Weekly Tech+Bio Highlights #66: AI Antibody Startup Hits Unicorn Status
Merck & Nvidia Debut Open-Source Drug Model; Microsoft’s Spatial Proteomics, & First Non-Profit Gets FDA Approval for Gene Therapy
Hi! This is BiopharmaTrend’s weekly newsletter, Where Tech Meets Bio, where we explore technologies, breakthroughs, and cutting-edge companies.
If this newsletter is in your inbox, it’s because you subscribed, or someone thought you might enjoy it. In either case, you can subscribe directly by clicking this button:
🤖 AI x Bio
(AI applications in drug discovery, biotech, and healthcare)
🔹 AI recreates immune landscapes from routine slides — Microsoft Research, Providence, and UW introduce GigaTIME, an AI model that generates virtual spatial proteomics from standard H&E pathology images, enabling large-scale analysis of tumor-immune interactions across 14,000 cancer patients and uncovering key predictors of immunotherapy response.
🔹 AI designs minimal gene editors — Researchers from the Doudna lab and collaborators use an AI model informed by structure and evolution to design compact gene editors, demonstrating activity in human and plant cells.
🔹 PsiThera launches with AI–physics fusion for oral biologics — PsiThera debuts with a platform combining physics-based simulations and AI to develop oral drugs for inflammation and immunology, advancing a lead program to optimization in ~4 months using its QUAISAR design engine.
🔹 Recursion reports positive Phase 1b/2 results for an AI-discovered MEK1/2 inhibitor, showing 43% median polyp reduction at 12 weeks and durable 53% reduction 12 weeks post-treatment in patients with familial adenomatous polyposis, a condition with no approved therapies.
🔹 AI model vs. AlphaFold limits — A new preprint from the EVOLVED 2024 team introduces Plica-1, a scalable protein prediction model reportedly handling structures 8x larger than AlphaFold3, enabling ultra-fast predictions on standard hardware.
🔹 New benchmark compares AlphaFold3, Chai-1 & peers — Shared by Siqi Sun (Fudan University), FoldBench is a new large-scale benchmark evaluating all-atom structure prediction models like AlphaFold3, Boltz-1, and Chai-1 across 1,522 biomolecular assemblies; while AF3 leads overall, antibody-antigen and ligand docking tasks remain key challenges.
🔹 LatchBio launches agent.bio, a public sandbox showcasing AI agents for spatial biology data analysis across leading platforms like MERFISH and Visium.
🔹 AI-native PACS debuts at RSNA — French startup Raidium unveils a PACS viewer powered by its Curia foundation model, trained on over 1B images to automate full diagnostic workflows across modalities, aiming to support radiologists with a single-prompt, end-to-end copilot.
This newsletter reaches over 10K industry professionals from leading organizations across the globe. To reserve your sponsor slot in one of the upcoming issues, contact us at info@biopharmatrend.com
🚜 Market Movers
(News from established pharma and tech giants)
🔹 Merck and Nvidia debut open-source drug model — Merck teams up with Nvidia to launch KERMT, a multitask AI model trained on 11M+ molecules to predict small-molecule drug properties.
🔹 AI meets immunology — Novartis partners with Relation to discover novel targets for atopic diseases, combining Relation’s AI platform with Novartis’s immuno-dermatology expertise to accelerate development of first-in-class therapies for immune-driven allergic conditions.
🔹 GSK spins out bioelectronic VC arm — GSK separates from Action Potential Venture Capital, its unit focused on electrical pulse-based therapies, with former leadership launching the new Synapse Fund—marking GSK’s second corporate VC spinout after SR One.
💰 Money Flows
(Funding rounds, IPOs, and M&A for startups and smaller companies)
🔹 AI antibody startup hits unicorn status — OpenAI-backed Chai Discovery raises $130M in Series B, reaching a $1.3B valuation just months after its last round, with funding driven by momentum around its zero-shot antibody design platform, capable of generating novel high-affinity binders for hard-to-drug targets.
🔹 Valinor Discovery, launched in May 2025, secures $13M to expand its ML platform, which uses multi-omic and clinical data to predict patient response and design more targeted clinical trials, aiming to reduce failure rates and accelerate drug development.
🔹 AI pharma adds CNS-targeting asset — Formation Bio acquires global rights (ex-China) to a CNS-penetrant TYK2 inhibitor from Lynk Pharmaceuticals, aiming to begin Phase 1 in 2026 via its new subsidiary, with deal value up to $605M.
🔹 AI-powered lab automation startup lands $52M — Medra raises a $52M Series A to scale its “Physical AI Scientist” platform, which combines robotic instrument control and autonomous experimental reasoning.
🔹 Apple Tree Partners files for bankruptcy to keep biotechs afloat & to maintain funding for 13 portfolio companies amid a dispute with key investor Rigmora.
🔹 First nonprofit-led gene therapy wins FDA approval — Italy’s Fondazione Telethon becomes the first nonprofit to gain FDA approval for a gene therapy, with Waskyra treating Wiskott-Aldrich syndrome using a patient’s own genetically corrected stem cells, following strong reductions in infections and bleeding in clinical studies.
🔹 EpilepsyGTx raises $33M for targeted seizure gene therapy — UK spinout secures $33M to advance an AAV-based gene therapy delivered directly to seizure hotspots in focal refractory epilepsy, aiming to achieve lasting seizure control with a single, localized treatment.
🔹 Lumexa Imaging raises $462.5M in IPO — North Carolina-based Lumexa Imaging more than doubles its target, pulling in $462.5M in its Nasdaq debut, as the radiology provider touts AI-driven gains across its 184-site imaging network and eyes a $140B U.S. diagnostic market.
🔹 Philips acquires AI heart imaging startup SpectraWAVE to integrate its AI-driven coronary imaging tools into its image-guided therapy platform.
⚙️ Other Tech
(Innovations across quantum computing, BCIs, gene editing, and more)
🔹 Neuralink hires key FDA brain-device regulator — Neuralink brings on David McMullen, former FDA official who oversaw neurological device approvals, as head of medical affairs, aiming to streamline regulatory pathways for its BCI as it targets commercial use by 2030.
🔹 Rethinking drug safety — Inductive Bio, Amgen, and academic partners launch a $21M ARPA-H-funded initiative to build AI models that predict drug toxicity using human organoids, with Amgen aiming to submit the first FDA application based on human data instead of animal testing.
🔹 Medable and Tufts CSDD launch Innovation Evidence Workshop series, sharing data linking decentralized trial components to 40% faster enrollment and 3x higher recruitment.
🔹 Qubit Pharmaceuticals and Q-CTRL demonstrate quantum-powered hydration-site prediction on IBM hardware, achieving classical-level precision with 123-qubit runs in 25 minutes.
🏛️ Bioeconomy & Society
(News on centers, regulatory updates, and broader biotech ecosystem developments)
🔹 Radiology dominates FDA AI approvals in 2025 — Thomas Hagemeijer of HGM Advisory reports how FDA-cleared AI algorithms grew steadily in 2025, with radiology making up 77% of approvals, while neurology gained ground; despite strong growth (46% CAGR since 2015), clinical adoption still lags due to performance and workflow limitations.
🔹 New York Gastroenterology Associates adopts Proscia’s AI-powered digital pathology platform to boost diagnostic speed and accuracy.
🔹 FDA qualifies first AI tool for MASH trials — PathAI’s AIM-NASH becomes the first FDA-qualified AI tool to assess liver biopsies in MASH clinical trials, aiming to standardize scoring of fat, inflammation, and scarring.
🔹 NSF backs next-gen research orgs — The NSF launches its Tech Labs Initiative to fund independent, interdisciplinary research teams outside academia, aiming to fast-track breakthrough technologies with milestone-based funding and operational autonomy, and invites public feedback via a new RFI.
Designing Full-Length Antibodies
Chai Discovery, founded in 2024 and backed by OpenAI among others, has raised a $130M Series B at a reported $1.3B valuation, bringing total funding to $225M just four months after its previous $70M round. The financing follows the release of Chai-2, the company’s latest antibody design model, and the company says the capital will be used to build out a broader computer-aided molecular design suite.
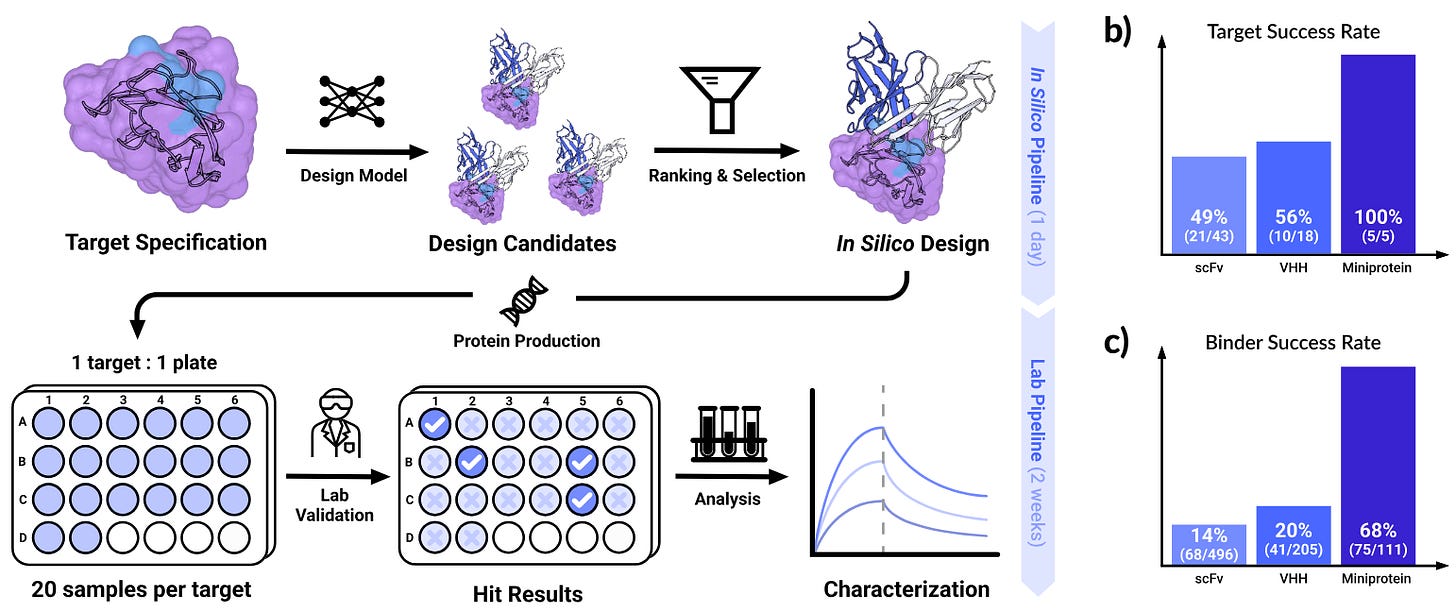
Chai-2 designs full-length monoclonal antibodies rather than fragments or binders alone. In internal studies cited by the company, 86% of generated antibodies showed zero or one flagged issue against common preclinical criteria, and 24 of 28 targets produced at least one candidate characterized as clean and drug-like. Structural validation using cryo-EM on five antibody–antigen complexes reportedly showed binding at intended epitopes with sub-angstrom agreement, including in CDR loops.
The company also reports results on harder targets. Chai-2 generated binders across six GPCRs while testing as few as 10 to 73 designs per target, including agonistic antibodies in full mAb format. In peptide–MHC experiments, testing 27 to 50 designs yielded binders specific to KRAS G12V, without binding to closely related variants. Chai frames its longer-term objective as producing IND-ready biologics in a single in silico pass, alongside a stated “Responsible Deployment policy” that restricts access based on use and safety considerations.
Microsoft’s Spatial Proteomics
A recurring limitation in immuno-oncology is that immune cell states in the tumor microenvironment matter, but are largely invisible in standard H&E slides. Methods that expose those states, such as multiplex immunofluorescence, are informative but expensive and difficult to apply broadly, which limits population-level analysis.
A recent paper in Cell from Microsoft Research, working with Providence and the University of Washington approaches this by treating routine pathology as a latent source of cell-state information. The authors build on the observation that H&E morphology and spatial organization already reflect underlying biological states, even if those states are not directly measurable in standard slides. GigaTIME is designed to translate these routinely available images into virtual multiplex immunofluorescence signals, estimating spatial proteomic patterns that are otherwise costly and scarce.
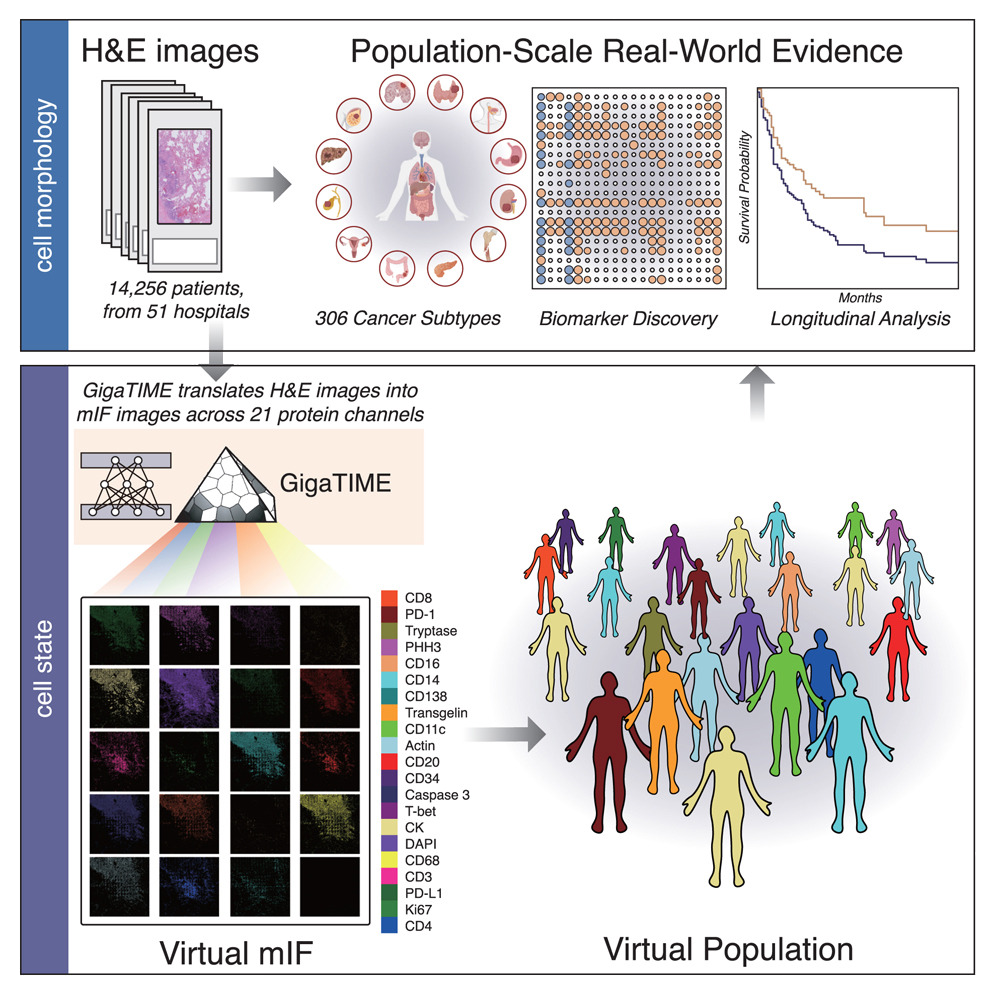
The team trained a model to translate H&E slides into virtual multiplex immunofluorescence signals and then used those simulated readouts to explore immune-related patterns across large patient cohorts. The work is positioned as a way to enable population-scale tumor microenvironment analysis that would otherwise be constrained by data availability, rather than as a replacement for physical assays or a complete account of immunotherapy response.
Read also:
Three Big Ideas in Aging Research That Could Shift the Therapeutic Landscape




Impressive how Chai-2 is tackling full-length mAbs rather than just fragments. The 86% success rate on preclinical criteria is solid, but the real test will be how those cryo-EM-validated structures actually perfrom in vivo. I've seen too many computationally-designed binders fail once they hit wet lab conditions. Still, if they can really push targets like GPCRs and peptide-MHC complexes with such low candidate counts, that changes the economics of early discovery substantially.