Weekly Tech+Bio Highlights #56: Rearranging Human DNA at Megabase Scale
Also: RFdiffusion3; gene therapy slows Huntington’s by 75%, & robots charting chemical hyperspace
Hi! This is BiopharmaTrend’s weekly newsletter, Where Tech Meets Bio, where we explore technologies, breakthroughs, and cutting-edge companies.
If this newsletter is in your inbox, it’s because you subscribed, or someone thought you might enjoy it. In either case, you can subscribe directly by clicking this button:
🤖 AI x Bio
(AI applications in drug discovery, biotech, and healthcare)
🔬 In a new bioRxiv preprint, researchers from the Baker Lab present RFdiffusion3, a unified diffusion model that generates protein structures with atom-level precision for tasks like enzyme catalysis and DNA binding, showing improved performance over earlier methods with lower computational cost.
🔬 BenchSci partners with Thermo Fisher to develop AI-powered software for experimental design, reagent selection, and literature analysis, aiming to boost R&D productivity across life sciences labs using BenchSci’s ASCEND platform.
🔬 Faster nanobody design with AI — Researchers from the Hie and Gao labs, in collaboration with the Arc Institute, unveil Germinal, an open-source AI framework that combines AlphaFold-Multimer and a language model to design functional nanobodies with nanomolar affinities using minimal experimental screening.
🔬 AI vs. rare oncology — Healx partners with Vuja De Sciences to expand its rare cancer pipeline, integrating Vuja’s osteosarcoma program and naming its founder to a leadership role, as HLX-4310 prepares to enter clinical trials backed by Healx’s AI-driven discovery platform.
🔬 Nature Reviews Bioengineering paper from George Church and colleagues presents a practical roadmap for integrating AI into protein design workflows, highlighting how generative and predictive tools are accelerating therapeutic and synthetic protein development.
🔬 AI replaces traditional stains — Proscia adds Pictor Labs’ virtual staining tech to its platform, enabling digital H&E, IHC, and special stains from unstained slides, cutting costs and preserving tissue without chemical processing.
🔬 AI meets aging biology — MindWalk (formerly IPA) advances an AI-designed GLP-1 longevity program combining receptor agonists with a second therapeutic targeting cellular resilience, aiming for chronic, preventive use to extend healthspan.
🔬 AI tackles glioblastoma complexity — NetraMark partners with a U.S. medical center to apply its explainable AI platform to glioblastoma CSF proteomics, aiming to define patient subgroups for trial design, biomarker discovery, and treatment stratification.
🔬 AI isn’t replacing radiologists — as reported in Works in Progress, despite hundreds of FDA-cleared imaging models and major advances in diagnostic AI, real-world use remains limited, with human radiologists in higher demand than ever due to performance gaps, legal hurdles, and the complexity of clinical workflows.
🔬 Toward a drug response atlas for the lung — Helmholtz Munich and Parse Biosciences partner to map how healthy and diseased human lung tissues respond to 900 drugs at single-cell resolution, using Parse’s GigaLab platform to build the largest lung tissue perturbation dataset to date.
🚀 Formation Bio adds former execs from Pfizer, BenevolentAI, and Incyte to accelerate its AI-driven drug development model.
🔬 Protein switches for precise timing — Researchers from the Baker Lab (again) report in Nature a general method for designing proteins with tunable dissociation kinetics, enabling rapid shutoff of signaling—demonstrated by switchable IL-2 mimics that disengage from receptors within seconds to control immune activation.
🚜 Market Movers
(News from established pharma and tech giants)
🔬 Merck expands its partnership with Variational AI into a deal worth up to $349M to develop small-molecule drugs for two challenging targets, leveraging Variational’s generative AI platform trained on hundreds of known targets.
💰 Sanofi commits $625M more to its venture arm, Sanofi Ventures, boosting AUM to over $1.4B to fund early-stage biotech and AI-driven health startups aligned with its R&D focus in immunology, rare diseases, neurology, and vaccines.
🔬 Streamlining oncology trials with AI — Pi Health partners with GSK to run a global Phase 2 oncology trial using its AI-powered clinical trial platform.
💰 Money Flows
(Funding rounds, IPOs, and M&A for startups and smaller companies)
💰 Manas AI, co-founded by oncologist Siddhartha Mukherjee and LinkedIn’s Reid Hoffman, raises a $26M seed extension to grow its full-stack AI drug discovery platform and names former Meta and Google exec Ujjwal Singh as CTO.
💰 NVIDIA backs UK AI surge, committing £2B to support AI startups across the UK, including Oxford-based ventures, in partnership with leading VC firms, marking what it calls the “big bang” of a new industrial revolution and the UK’s largest AI infrastructure investment to date.
🚀 Moderna opens £1B U.K. mRNA hub — launching its Innovation and Technology Centre in Harwell with capacity for 100M vaccine doses annually, expanding to 250M in a pandemic, anchoring a £1B+ R&D and manufacturing investment to bolster the U.K.’s life sciences sector.
⚙️ Other Tech
(Innovations across quantum computing, BCIs, gene editing, and more)
🔬 Gene therapy milestone in Huntington’s — uniQure reports Phase I/II success for its experimental gene therapy, showing a 75% slowing of disease progression over 36 months, paving the way for a 2026 FDA filing and potential launch.
🔬 Large-scale genome rewriting — Arc Institute and collaborators report, now in Science, programmable bridge recombinases that insert, delete, or invert up to ~1 Mb of human DNA without relying on repair pathways, showing up to 20% efficiency and enabling edits like repeat excision and enhancer deletion for disease modeling.
🔬 Tempus wins FDA clearance for NGS assay — Tempus AI receives 510(k) clearance for its xR IVD device, an RNA-based sequencing assay to detect gene rearrangements in solid tumors, supporting oncology drug development and clinical decision-making.
🔬 In vivo CAR-T — Aera Therapeutics reports preclinical data on AERA-109, a lipid nanoparticle–delivered in vivo CAR-T therapy that generates engineered T cells inside the body without viral vectors or cell harvesting, targeting B cell–driven autoimmune diseases.
🔬 Electricity-free hearing tech — Researchers report an artificial cilia-based device that decodes sound frequencies (100–6,000 Hz) without electricity, using acoustic resonance for tasks like drug release, with potential applications in personalized hearing and bio-interfacing.
💰 Biogen has officially ended all AAV-based gene therapy programs, joining Roche, Takeda, and Vertex in walking away from the costly, single-use modality, and will reallocate resources toward higher-probability treatment platforms, with minor layoffs reported.
🔬 Robots chart chemical hyperspace — a low-cost, robot-assisted platform maps thousands of reaction conditions using optical detection, revealing unexpected reactivity, switchable products, and hidden intermediates, potentially offering a scalable framework for reaction discovery and optimization.
🔬 Chips that predict chemo response — team from McGill and Harvard developed a patient-specific organ-on-a-chip that accurately predicted esophageal cancer responses to chemotherapy in just 12 days.
🚀 A New Kid on the Block
(Emerging startups with a focus on technology)
💰 UK startup brings adaptive AI to drug R&D as DaltonTx emerges from stealth with £4M seed funding to build an AI platform that integrates into pharma workflows, combining human input and machine learning to accelerate discovery across small molecules and biologics.
Rearranging Human DNA at Megabase Scale
You might remember last week’s preprint from Arc Institute and Stanford, where a fine-tuned LLM (Evo 2) generated hundreds of genomes, 16 of which replicated and killed E. coli in the lab. That was synthetic virology at genome scale.
This week, Arc is back with a Science paper, where they report that “bridge editing” now works in human cells, enabling insertions, deletions, and inversions across regions up to ~1 Mb.
The group optimized a bacterial recombinase system (ISCro4) and its guide RNA to reach ~20% insertion efficiency with ~82% on-target specificity, then demonstrated edits including removal of expanded repeats in a Friedreich’s ataxia model construct and disruption of the BCL11A enhancer.
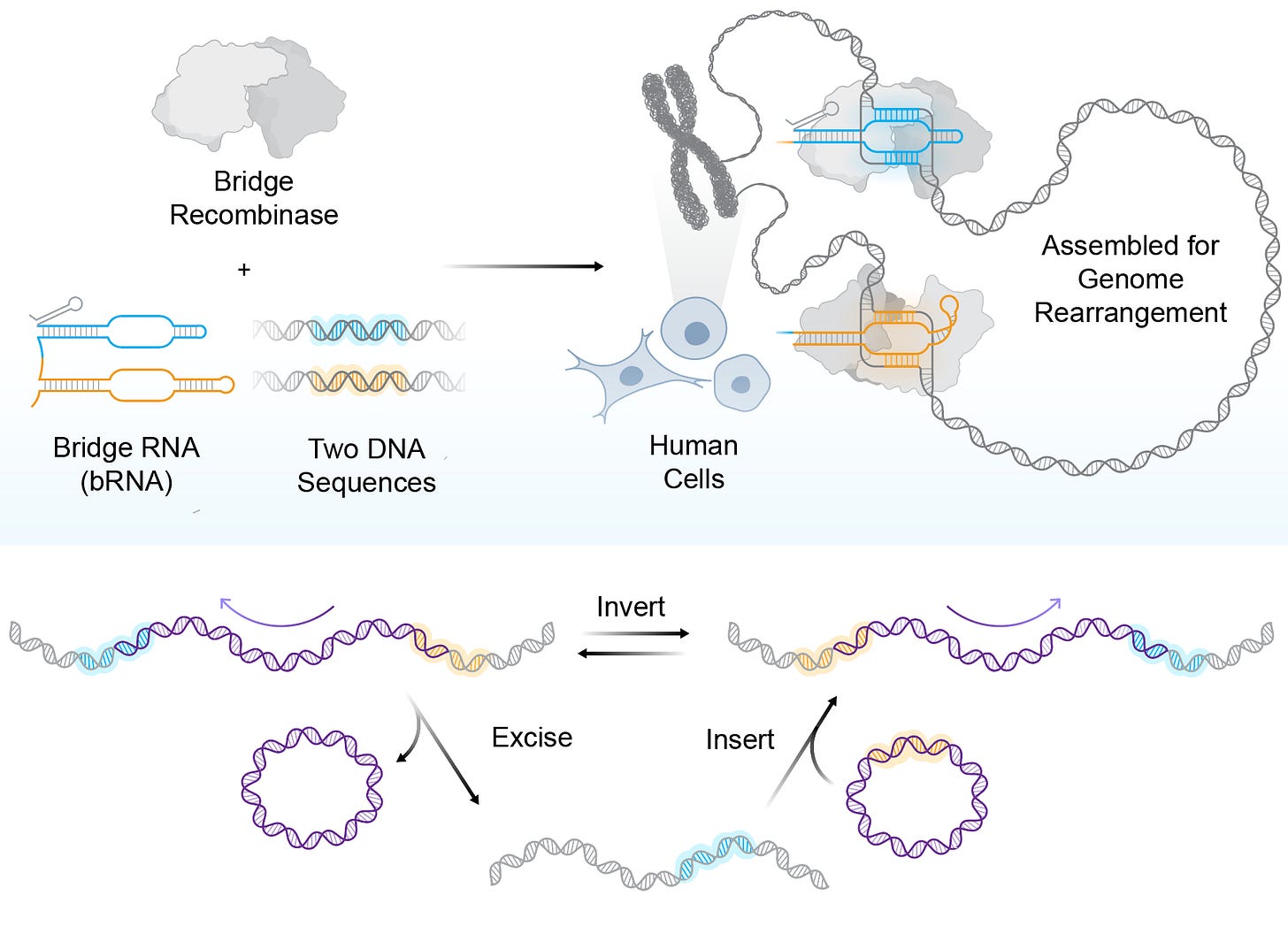
The system was first described in Arc’s May 2025 preprint, where bridge recombinases pair a recombinase enzyme with a structured “bridge RNA” that recognizes two DNA sites at once, bringing distant loci together so the enzyme can cut-and-paste, excise, or flip sequence segments in a single programmed step. The team previously showed bridge recombination in bacteria; today’s paper extends it to human cells.
The screen started with 72 natural systems from bacteria. Roughly a quarter showed activity in human cells, but only one (ISCro4) was strong enough to warrant engineering. After systematic protein and RNA guide optimization across thousands of variants, the group reports ~20% insertion efficiency and ~82% specificity at targeted human genomic sites.
Mechanistically, the dual-targeting bridge RNA is the key difference from CRISPR’s single-site guide. By tethering two genomic addresses, the system supports coordinated rearrangements: bringing sites together to excise intervening DNA, inverting a defined segment, or inserting a payload at a programmed location.
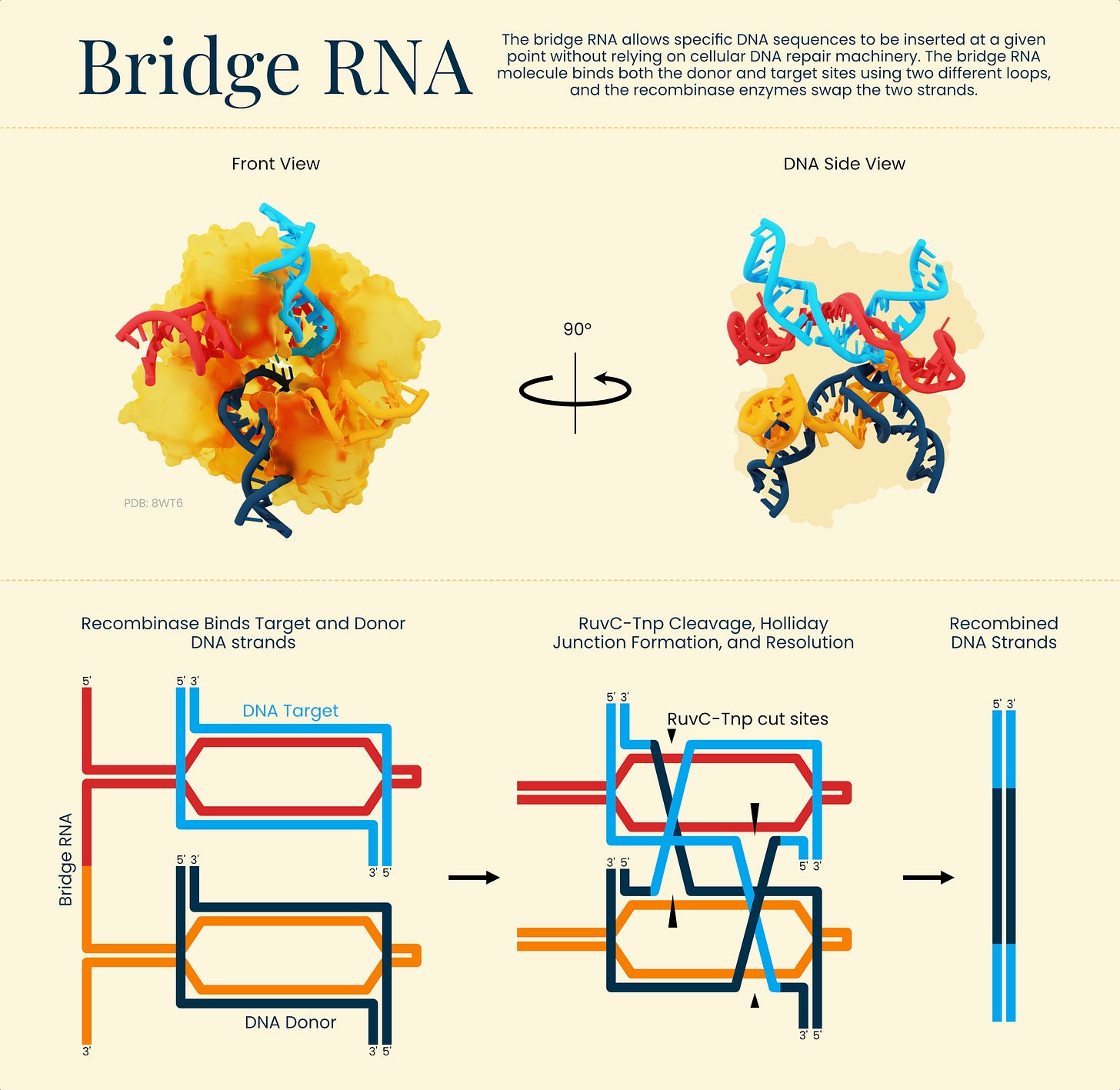
As demonstrations, the authors built constructs carrying long repeat expansions that model Friedreich’s ataxia and used ISCro4 to remove the repeats, in some cases eliminating more than 80% of the expanded tracts in the construct context. They also targeted and removed the BCL11A enhancer, an established therapeutic edit.
Planned work includes testing in immune and stem cells, developing delivery approaches, and engineering variants aimed at segments larger than one megabase. The group also notes potential applications in plant genetics, synthetic biology, and modeling large cancer-associated rearrangements.
Materials are being made available for labs: plasmids via Addgene and a bridge RNA design tool that returns sequences for any two user-specified DNA targets, according to the authors.