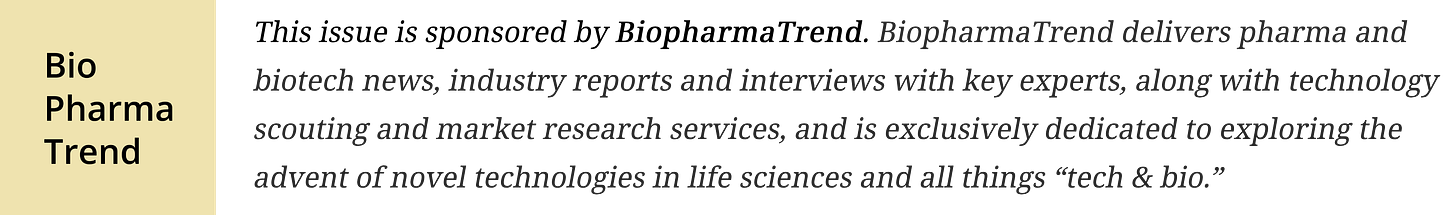Weekly Tech+Bio Highlights #54: Era of Generative Genomics
Emerging Unicorn in Autonomous AI Labs, Benchmarking Scientific AI Agents, & Hitting "Undo" on Disease
Hi! This is BiopharmaTrend’s weekly newsletter, Where Tech Meets Bio, where we explore technologies, breakthroughs, and cutting-edge companies.
If this newsletter is in your inbox, it’s because you subscribed, or someone thought you might enjoy it. In either case, you can subscribe directly by clicking this button:
🤖 AI x Bio
(AI applications in drug discovery, biotech, and healthcare)
🔹 AI-guided cancer drug shows early promise — Lantern Pharma’s LP-184 met all primary endpoints in a Phase 1a trial, showing strong safety and signs of antitumor activity in hard-to-treat solid tumors, including glioblastoma and NSCLC, with 48% of patients at therapeutic doses achieving disease control.
🔹 Cracking the black box of disease biology — BenchSci unveils LENS, an AI system designed to extract structured, evidence-based findings from biomedical papers across fields, achieving over 90% completeness and <1% hallucination by leveraging a "universal scientific grammar"; the system underpins a Biological Evidence Knowledge Graph aimed at supporting neuro-symbolic reasoning and reducing failure in drug development.
🔹 Causaly launches agentic AI for life sciences R&D — Agentic Research is a domain-specific AI platform combining internal and external biomedical data with multi-agent systems to automate hypothesis generation, literature analysis, and decision-making across drug discovery workflows.
🔹 Medra launches its AI-powered Continuous Science Platform combining robotics and multimodal AI to automate and optimize lab experiments, aiming to accelerate discovery in areas like antibody engineering and gene therapy.
🔹 New AI model predicts combinations that reverse disease states — Researchers at Harvard (Marinka Zitnik) and collaborators introduce PDGrapher, a causally inspired graph neural network that identifies gene or drug perturbations capable of shifting diseased cells toward healthy states, outperforming existing methods in accuracy and speed across 19 datasets and 11 cancer types.
🔹 Bioptimus assembles advisory board for AI-driven biology — The French startup, founded in 2024 by ex-Google DeepMind and Owkin scientists, has named top experts in genomics, systems biology, and AI to guide development of its universal foundation model integrating multi-scale biological data for research and medicine.
🔹 Better tokens for better antibodies — Researchers at Prescient Design and Google DeepMind (Ada Fang) introduce Igloo, a multimodal tokenizer for antibody loops that improves structure-aware antibody modeling and design, outperforming existing methods in retrieving, predicting, and generating critical CDR H3 loops for biologics development. Available on GitHub.
🔹 How Good Are AI Scientists? — Researchers (Henry E. Miller, Matt Greenig, Benjamin Tenmann and Xaira’s Bo Wang) release BioML-bench, a benchmark suite for evaluating AI agents on end-to-end biomedical machine learning tasks across drug discovery, imaging, omics, and protein engineering; results show agents underperform human experts, with success tied more to flexible strategies than domain specialization.
🔹 Proscia expands AWS collaboration to scale AI pathology — integrating its cloud-native pathology platform with AWS HealthImaging.
🔹 Tempus AI validates its RNA-based PurIST algorithm as a prognostic and predictive biomarker for guiding first-line chemotherapy in advanced pancreatic cancer, showing improved survival in classical-subtype patients treated with FOLFIRINOX.
🔹 Eli Lilly and insitro will co-develop machine learning models to predict small molecule pharmacology using Lilly’s proprietary ADMET data, aiming to accelerate hit-to-lead efforts and reduce reliance on in vivo studies via the TuneLab federated AI platform.
🔹 Absci partners with Oracle and AMD to scale its AI drug discovery platform using Oracle Cloud and AMD's Instinct MI355X GPUs, aiming to accelerate biologics design and reduce computational costs.
🔹 XtalPi and PharmaEngine receive regulatory clearance in Taiwan and Australia to begin Phase I trials of PEP08, an AI-designed second-generation PRMT5 inhibitor targeting solid tumors.
🚜 Market Movers
(News from established pharma and tech giants)
🔹 Novartis doubles down on molecular glue drugs — signs a second deal with Monte Rosa Therapeutics worth up to $5.7B, including $120M upfront.
🔹 Google Research unveils a Gemini-based system that generates reproducible research software using LLMs and tree search, outperforming expert baselines in genomics, COVID forecasting, and other scientific domains.
🔹 GLP-1 drugs now in trials for 100+ diseases — Phesi reports that GLP-1 therapies, traditionally used for diabetes and obesity, are being tested across over 100 conditions including cardiovascular disease, PCOS, and cancers, based on analysis of 583 ongoing trials and patient data from nearly 2 million records.
🔹 Lung disease proposed as fast-track model for anti-aging drugs — A study from Insilico Medicine, Buck Institute, and Duke University highlights idiopathic pulmonary fibrosis (IPF) as a high-potential model for evaluating geroprotective therapies, citing its rapid progression and overlap with aging biology as key advantages for accelerating drug discovery.
💰 Money Flows
(Funding rounds, IPOs, and M&A for startups and smaller companies)
🔹 Lila Sciences raises $235M in Series A funding, reaching unicorn status to scale its AI Science Factories—autonomous labs combining AI, robotics, and software to automate the full scientific method across life sciences, materials, and energy.
🔹 GE HealthCare to acquire Belgium-based icometrix to integrate its AI-driven brain MRI platform, icobrain, into GE’s imaging systems, enhancing neurology diagnostics, including Alzheimer’s-related ARIA detection.
🔹 China’s robotic surgery market heats up — Ronovo Surgical raises $67M in Series D funding backed by J&J to scale its modular laparoscopic robot platform, now approved in China for multiple surgical specialties, with over $100M raised in 2025.
⚙️ Other Tech
(Innovations across quantum computing, BCIs, gene editing, and more)
🔹 datma launches a privacy-preserving Federated Biomarker Explorer for Health Systems, enabling hospitals and labs to assess pharma interest in their data without sharing patient records or committing resources.
🔹 Smart catheter gains EU approval — Neurescue’s automated balloon occlusion device earns CE Mark to treat non-shockable cardiac arrest, using sensor-guided inflation to boost blood flow to the heart and brain without the need for imaging.
🔹 Philips and Masimo deepen AI-powered monitoring to integrate advanced wearable sensors and AI tools into bedside monitors, aiming to enhance remote and in-hospital patient monitoring.
🔹 Smarter surgery through software — Intuitive adds real-time force feedback and in-procedure video replays to its robot, leveraging more computing power and support future AI-driven updates.
🚀 A New Kid on the Block
(Emerging startups with a focus on technology)
🔹 Multimodal AI for whole-patient understanding — Sophont’s 22-year-old founder Tanishq Abraham (ex-Stability AI), raises $9.22M to build foundation models that integrate imaging, text, and lab data for clinical decision support, aiming to redefine infrastructure for healthcare AI. Backed by Kindred Ventures, Google Chief Scientist Jeff Dean, Hugging Face’s Clément Delangue, Weights & Biases co-founder and CEO Lukas Biewald and others, Sophont is building an open, modular AI system to discover new biomarkers and support multispecialty diagnostics; its founders, with backgrounds in neuroimaging and medical AI research, plan to release state-of-the-art unimodal models ahead of a full multimodal system.
🔹 AI replaces the wet lab? — Synthesize Bio debuts from stealth with GEM-1, a generative AI model that simulates gene expression from experimental inputs with lab-level accuracy, aiming to speed up and replace costly RNA-seq experiments; backed by $10M seed funding, the startup is led by Jeff Leek and Robert Bradley. A new preprint shows GEM-1 can predict future gene expression data with accuracy on par with real lab experiments, including bulk and single-cell RNA-seq; the platform enables researchers to generate, explore, and analyze synthetic datasets via API or web interface.
🔹 AI meets enzymes for next-gen precision drugs — Ridge Biotechnologies emerges from stealth with $25M in seed funding to develop AI-driven platforms for enzyme and prodrug design, enabling safer, more targeted therapies including ADCs and in vivo cell therapies. Founded by ex-SwiftScale CTO Weston Kightlinger and incubated by Sutter Hill Ventures, Ridge combines cell-free experimentation with proprietary ML to generate massive sequence-function datasets, powering tools like NativeLink and ProTrigger for modular, high-specificity bioconjugation and targeted payload release.
This newsletter reaches over 9.6K industry professionals from leading organizations across the globe. Reserve your sponsor slot in one of the upcoming issues.
Contact us at info@biopharmatrend.com
Emerging Unicorn in Autonomous AI Labs
Lila Sciences closed a $235M Series A, valuing the Cambridge startup at over $1B less than a year after launch, and bringing total funding to ~$450M. The company describes “AI Science Factories” that pair models, simulations, and robotics to generate hypotheses, design experiments, run them, and learn from results; it says the first factory has run hundreds of thousands of AI-driven experiments across life sciences, chemistry, and materials. Leadership includes Geoffrey von Maltzahn, with George Church as Chief Scientist.
The pitch fits a broader push toward autonomous labs—variants are being tested by groups like Insilico Medicine, Ginkgo Bioworks with partners, and newer efforts such as Potato. As our analysis notes, capital and throughput are rising, but the key metric remains downstream outcomes; late-stage clinical wins from AI-discovered programs are still limited.
Read the full coverage by Andrii Buvailo on our site for details on investors, applications, and how Lila’s “scientific superintelligence” vision compares with other models.
Enter “Generative Genomics”
Synthesize Bio came out of stealth with GEM-1, described as a model that predicts the outcome of gene-expression experiments from a plain-English setup—cell type, perturbation, context—and returns a synthetic RNA-seq profile that’s meant to match what a lab would measure. The company was founded by Jeff Leek and Robert Bradley, based in Seattle, with $10M seed in 2023 and a ~15-person team.
GEM-1 is trained on a very large curated RNA-seq corpus—tens of thousands of profiles across conditions, cell types, and perturbations. Inputs are textual descriptions of the experiment (e.g., knockouts, cell type, treatment). The output is a synthetic gene-expression profile intended to mirror what one would measure. The claim is that this could replace many wet-lab runs with seconds-scale simulation and that the model can be adapted to specific disease settings as a foundation layer.
According to the preprint and launch coverage, GEM-1 reports agreement with strong lab benchmarks and frames the approach as “generative genomics”—models generating RNA-seq readouts from experimental designs rather than measuring them. Madrona characterizes early “super-experimental” results and presents GEM-1 as a foundation that can be adapted to disease contexts, cell lines, or therapeutic areas.
Access is listed as available via R and Python clients and a Synthesize.bio workspace.
How good are AI scientists?
Short answer—capable, but below expert level.
Henry E. Miller (Shift Bioscience), Matthew Greenig (Cambridge/ScienceMachine), Benjamin Tenmann (ScienceMachine), and Bo Wang (University of Toronto/Vector Institute/Xaira Therapeutics) release a benchmark for agent performance on end-to-end biomedical ML across protein engineering, single-cell omics, biomedical imaging, and drug discovery.
Tasks ship as “capsules” with data and scoring; agents run in Docker and are graded by metrics like AUROC and Spearman. The suite borrows mechanics from MLE-bench and draws tasks from ProteinGym, OpenProblems, and PolarisHub.
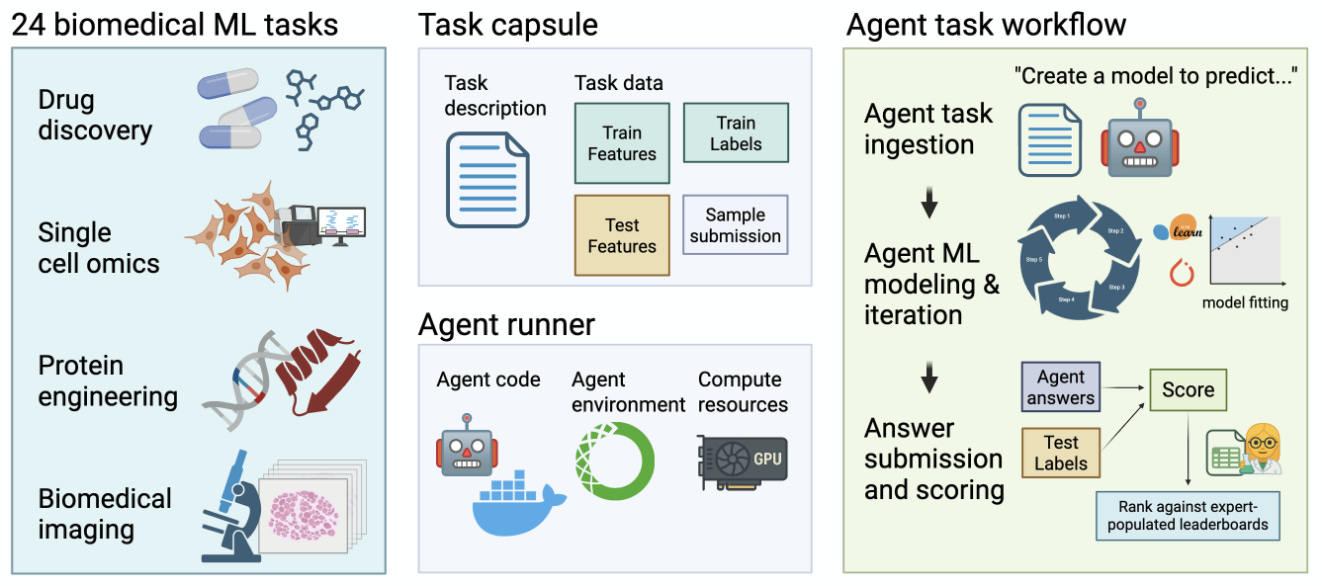
According to the paper, four open-source agents were tested—STELLA and Biomni (biomedical specialists), AIDE and MLAgentBench (generalists). All underperformed human baselines on average; reported placements clustered around the mid-30s percentiles.
The paper also notes that base LLM choice can blur comparisons, so the team paired each agent with its recommended model and plans to vary LLMs more systematically. Human leaderboards are used as a pragmatic reference, with a suggestion to add automated baselines like H2O AutoML and Lazy Predict. Next iterations aim to broaden tasks and agents, strengthen scaffolding for deep learning, and probe tool-use, memory/self-evolving behaviors, and multi-agent designs. Materials are open-source with code at GitHub.
Hitting "Undo" on Disease
Harvard’s Marinka Zitnik and collaborators introduce PDGrapher, a graph neural network that tackles the inverse problem in phenotype-driven discovery: instead of predicting how a perturbation changes a cell, it proposes gene or drug perturbations that move a diseased expression state toward a treated/healthy one. It represents disease states on gene networks and learns which targets to hit so the shifted profile matches what’s seen in treatment data.
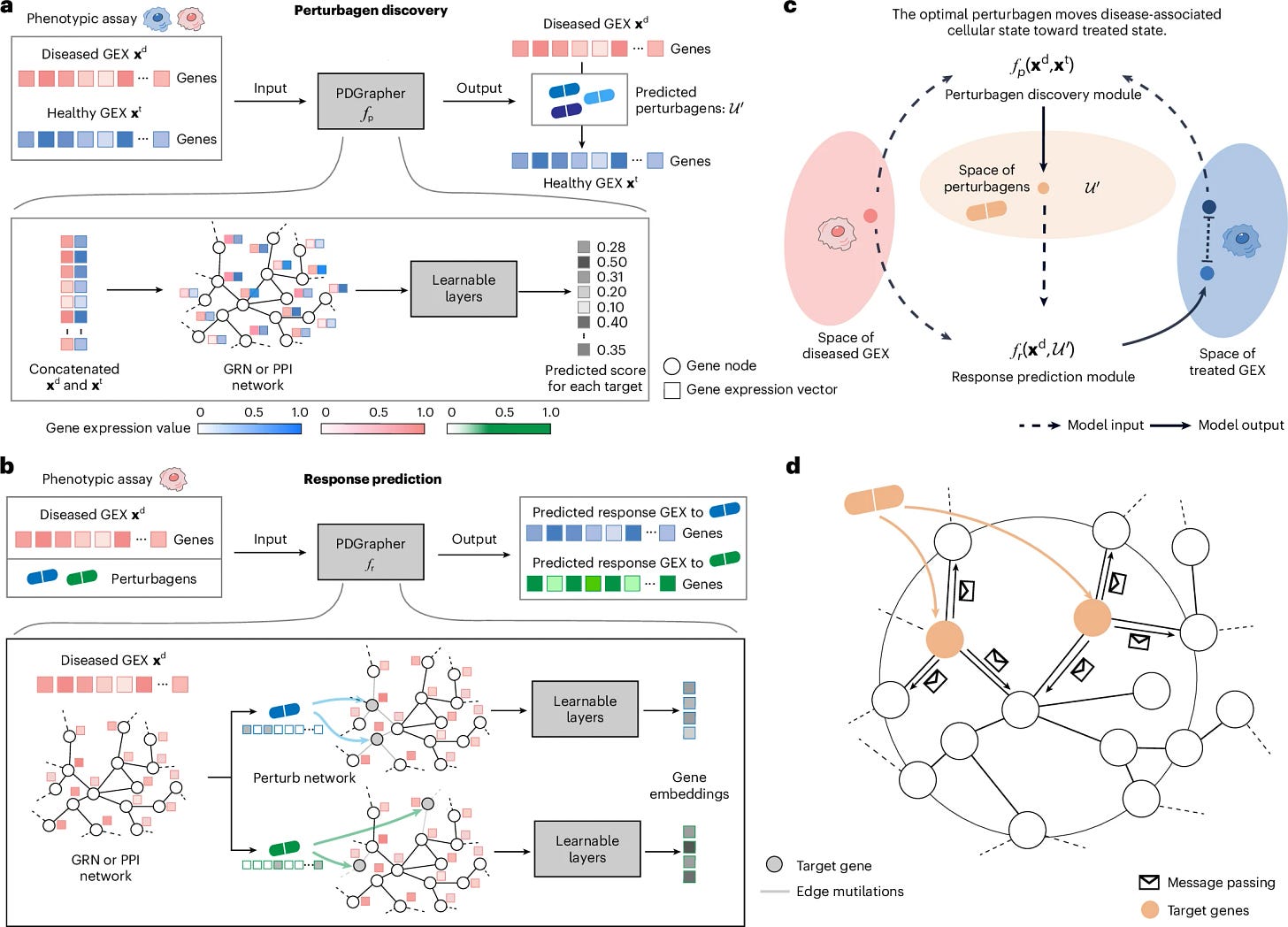
According to the paper, across 19 datasets and 11 cancer types, PDGrapher recovers true drug targets with higher recall than competing methods in held-out tests (up to +13.4% on chemical datasets), improves ranking of ground-truth targets (+0.13 nDCG), identifies combinatorial interventions rather than just single targets, and trains faster (reported up to 25x) because it predicts targets directly instead of simulating every possible perturbation.
Code is available, and the authors outline extensions they plan to explore—wider task settings, stronger support for deep models, and checks that separate model design effects from the underlying base model.