Weekly Tech+Bio Highlights #51: Neurosymbolic AI for Biomedical Research
$6B AI Drug Discovery Deal & FDA’s AI Lifecycle Concept for Health Care
Hi! This is BiopharmaTrend’s weekly newsletter, Where Tech Meets Bio, where we explore technologies, breakthroughs, and cutting-edge companies.
If this newsletter is in your inbox, it’s because you subscribed, or someone thought you might enjoy it. In either case, you can subscribe directly by clicking this button:
BiopharmaTrend case study is out examining one of the core challenges in biomedical R&D: the volume and fragmentation of scientific data. Millions of new articles, figures, and datasets appear each year in inconsistent formats and behind closed-access journals, making it hard to trace findings, resolve conflicting results, and especially to connect related evidence.
In this case study, we look at how Toronto-based BenchSci—a Google-backed company that began with machine learning tools for antibody selection and now works with many of the world’s top pharmaceutical firms—approaches the problem with ASCEND, a platform linking large language models to a structured knowledge graph of over 400 million entities and 1 billion relationships.
We examine how the neurosymbolic approach handles ambiguous shorthand, aligns studies that describe the same biology differently, and brings public and internal datasets into one frame. The aim is a system that reads, links, and audits evidence like a scientist—context-aware, source-traceable, and able to follow relationships across text, figures, and supplementary data.
This newsletter reaches over 9K industry professionals from leading organizations across the globe. Reserve your sponsor slot in one of the upcoming issues.
Contact us at info@biopharmatrend.com
🤖 AI x Bio
(AI applications in drug discovery, biotech, and healthcare)
🔹 Salk team’s AI reveals cancer-linked microproteins—Velia Therapeutics co-founder Alan Saghatelian and postdoc Brendan Miller develop ShortStop, a machine learning model trained on real and false microprotein genes to mine RNA-seq data, uncovering tumor-associated proteins that could be targeted with antibodies or other therapies.
🔹 AI speeds cancer trial enrollment—WVU Cancer Institute joins Ryght AI’s network to use generative and agentic AI, including site digital twins, to cut trial activation from months to weeks and streamline patient matching across its oncology programs.
💰 Money Flows
(Funding rounds, IPOs, and M&A for startups and smaller companies)
🔹 Tahoe Therapeutics raised $30M, boosting its valuation to $120M, to expand its Mosaic platform and build AI-powered virtual cell models using massive single-cell datasets, with the goal of accelerating cancer drug discovery.
🔹 AI super-deal in drug discovery—XtalPi and DoveTree sign a $6B agreement to co-develop therapeutics for hard-to-drug targets in oncology, immunology, neurology, and more, combining AI, quantum modeling, and robotics with DoveTree’s target validation expertise; includes $51M upfront, $49M near-term, and up to $5.89B in milestones and royalties.
🔹 Zero-shot AI for biologics—Chai Discovery raises $70M to scale its generative antibody design platform, achieving up to 20% hit rates on hard targets without templates, and boosting its valuation to $550M for partnerships in AI-driven therapeutic discovery.
🔹 Advancing programmable mRNA cancer therapies—Strand Therapeutics raises $153M in Series B to expand clinical trials for localized IL-12–expressing mRNA treatments, aiming to reprogram tumor environments and reduce systemic toxicity; funding boosts valuation to $550M and supports pipeline growth toward 2030 market entry.
🔹 Late-stage cardio tech—Orchestra BioMed secures $111M from a public offering and strategic investments by Medtronic and Ligand to advance trials of its blood pressure neuromodulation therapy and drug-delivering angioplasty balloon toward regulatory milestones.
🔹 Scaling up single-cell genomics—10x Genomics acquires Scale Biosciences for $30M plus milestones, adding its high-throughput sample barcoding technology to enable lower-cost, large-scale RNA sequencing and expand single-cell research capabilities.
🔹 Apreo Health raises $130M in Series B to advance clinical trials of its bronchoscopically delivered airway scaffold, aiming to improve lung function and quality of life for patients with severe emphysema.
🔹 ADC partnership ends early—Ipsen terminates its $875M agreement with Sutro Biopharma for a ROR1-targeting cancer therapy after reviewing new data and competitive developments, leaving Sutro without $800M in potential milestones but with cash runway projected to 2027.
🔹 AI heart imaging heads to market—HeartFlow ups its IPO target to nearly $300M to expand its FDA-cleared AI CT-scan platforms for coronary diagnostics and upcoming 3D planning tools for stent procedures.
🏛️ Bioeconomy & Society
(News on centers, regulatory updates, and broader biotech ecosystem developments)
🔹 Pushing non-animal testing to regulatory approval—the NIH Foundation launches the Validation and Qualification Network to accelerate acceptance of AI-based toxicology models, organoids, and organ-on-chip systems, uniting pharma, regulators, and CROs to refine late-stage methods and reduce reliance on animal testing.
🔹 Regulator’s return—biotech stocks fell after news that Vinay Prasad would return to lead the FDA’s biologics center, reviving concerns over his past confrontations with review staff and halted gene therapy shipments; Sarepta, Capricor, and Replimune shares dropped up to 13% according to Bloomberg.
💊 Pharma
🔹 AI-powered longevity drug discovery—BioAge Labs posts Q2 2025 results showing a $21.6M net loss on higher R&D spend, $313M cash runway to 2029—the company advanced an oral NLRP3 inhibitor toward Phase 1 for obesity, expanded high-potency APJ agonist portfolio, and launched large-scale molecular profiling of 17,000+ biobank samples to fuel its longevity-focused drug discovery platform.
🔹 Brain-penetrating Alzheimer’s antibody shows safety edge—Denali Therapeutics reports a transferrin receptor–enabled antibody that clears amyloid plaques in mice without signs of ARIA-related brain bleeding, aiming for human trials next year as a potentially safer alternative to first-generation Alzheimer’s drugs.
FDA Maps AI Lifecycle for Generative AI in Healthcare
The FDA’s Digital Health Center of Excellence (DHCoE) recently published a blog laying out an AI lifecycle (AILC) concept that maps standard software development lifecycle phases to AI development for medical devices. The authors argue for lifecycle management as the organizing approach, with emphasis on data and model evaluation up front and post‑deployment monitoring later, and invite the community to iterate on the model.
The authors, Troy Tazbaz and John Nicol, frame the problem as such: AI systems in health care adapt in the real world, which can improve performance, but also create risk like amplifying bias and harming underrepresented groups. They point to lifecycle management, long used in software engineering, as a way to keep safety, effectiveness, and trustworthiness in scope from planning to retirement.
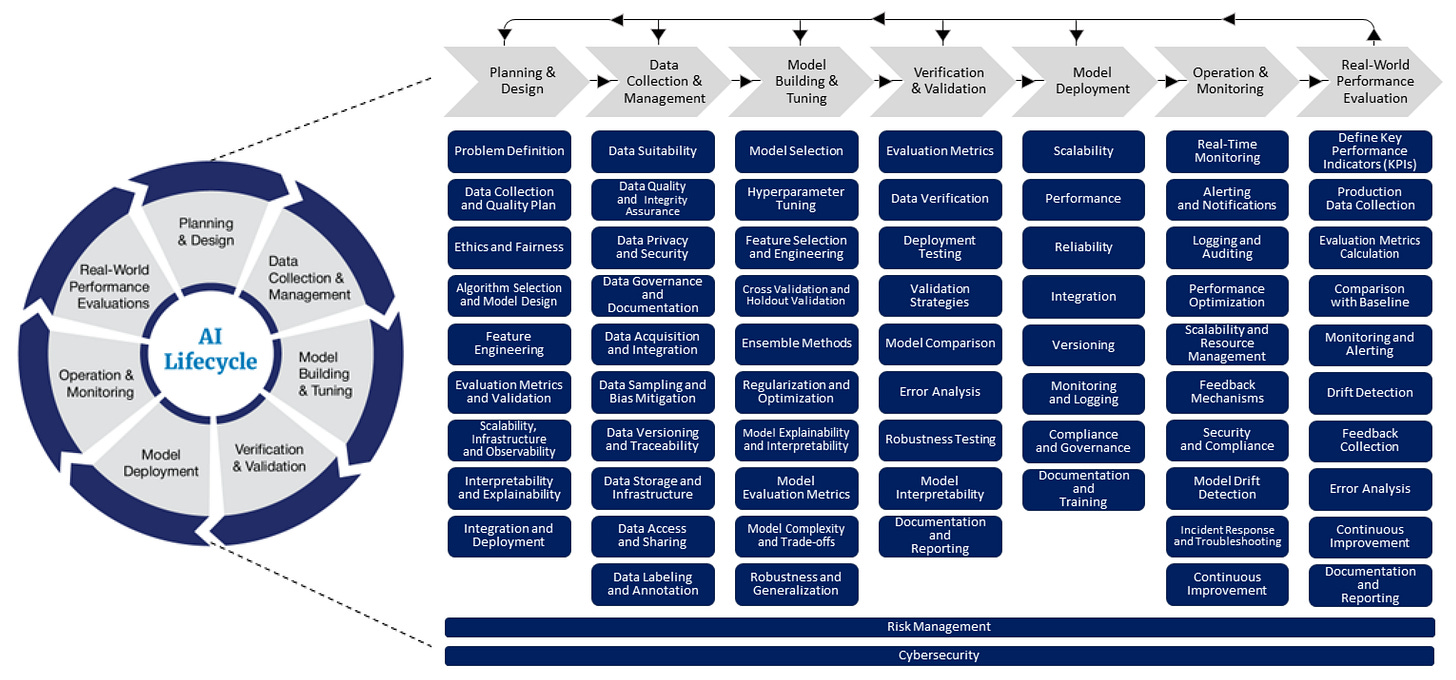
Their AILC sketch compiles activities from the literature and consensus standards, aligning them with phases such as Data Collection and Management, Model Building and Tuning, Operation and Monitoring, and Real‑World Performance Evaluation.
The example zooms in on “data suitability,” suggesting concrete evaluation axes like data quality, population coverage, and provenance, plus operational tooling for preprocessing, augmentation, and bias detection. Standards are positioned as guardrails for quality, interoperability, ethics, transparency, and compliance, and the AILC lens is presented as a way to spot gaps specific to medical devices and health care.
DHCoE proposes using the AILC as a practical playbook: build checklists for developers, ground model development in reliable and ethically sound data practices, and evaluate standards, tools, metrics, and best practices across the full lifecycle, including after deployment.





