Weekly Tech+Bio Highlights #50: How Robust Are Pathology Foundation Models?
CRISPR-GPT, Current Gene Delivery Landscape, & Thought-Controlled iPad
Hi! This is BiopharmaTrend’s weekly newsletter, Where Tech Meets Bio, where we explore technologies, breakthroughs, and cutting-edge companies.
If this newsletter is in your inbox, it’s because you subscribed, or someone thought you might enjoy it. In either case, you can subscribe directly by clicking this button:
Updates in AI-driven biotech and neurotech: Profluent published OpenCRISPR-1 in Nature, unveiling the first AI-generated Cas9 alternative with reduced off-target effects and releasing the largest CRISPR dataset to date. Stanford’s CRISPR-GPT achieved >80% editing efficiency using a multi-agent LLM system. OpenAI-backed Ambience Healthcare raised $243M to scale its clinical automation platform. Neuralink launched its first UK brain implant trial, while Synchron enabled iPad control via brain implant in ALS patients. Meanwhile, White House briefly froze all NIH funding before lifting the directive; the Senate has moved to increase NIH’s FY2026 budget by $400M.
🤖 AI x Bio
(AI applications in drug discovery, biotech, and healthcare)
🔹 Plain-language agent-based modeling for biology—in Cell, Johns Hopkins and University of Maryland researchers publish a “cell behavior grammar” that turns biological hypotheses into executable agent-based models, enabling biologists to simulate multicellular dynamics directly from transcriptomic data without coding.
🔹 Profluent unveils AI-designed genome editor in Nature—publishes OpenCRISPR-1, an AI-generated Cas9 alternative with reduced off-target effects and immunogenicity, and releases the CRISPR–Cas Atlas—the largest CRISPR dataset to date—marking a major step in LLM-driven protein design.
🔹 CRISPR-GPT streamlines gene editing design—Stanford-led study in Nature Biomedical Engineering presents CRISPR-GPT, a multi-agent LLM system trained on CRISPR literature and integrated with bio tools, enabling >80% editing efficiency in real-world multigene and epigenetic edits—even by novice users.
🔹 Lantern Pharma unveils predictBBB.ai, an open-access AI platform with 94% accuracy for blood-brain barrier permeability prediction, and completes early patient enrollment in Japan for its Phase 2 NSCLC trial targeting never-smokers.
🔹 Recursion details AI-driven infectious disease R&D—in ACS Infectious Diseases, it highlights how its AI platform accelerates therapeutic discovery, overcomes data scarcity in neglected diseases, and streamlines clinical trials through phenomics, generative design, and real-world patient data integration.
🔹 Nosis Bio advances AI-designed fibrosis drug—nominating its first development candidate for fibrosis, a cell-targeted gene silencer showing superior efficacy and safety in preclinical studies.
🔹 Simple models rival deep learning in gene perturbation prediction—a Nature Methods study finds that linear baselines often match or outperform foundation and deep learning models in predicting transcriptomic responses, highlighting limits in generalization and the need for task-specific model selection.
🔹 Neural networks predict early disease onset—University of Westminster researchers used deep learning on UK Biobank data to forecast early onset of 38 age-related diseases, identifying high-risk individuals and clusters of conditions for earlier personalized intervention.
🔹 AI tool streamlines protein fusion—ProDomino, trained on 170K natural insertions, predicts fusion-compatible sites to accelerate design of responsive protein tools like light- and drug-controlled genome editors.
This newsletter reaches over 9K industry professionals from leading organizations across the globe. Reserve your sponsor slot in one of the upcoming issues.
Contact us at info@biopharmatrend.com
🚜 Market Movers
(News from established pharma and tech giants)
🔹 Anthropic joins CMS health data pledge, committing its conversational AI tools to improve healthcare data interoperability and patient access, leveraging its Model Context Protocol to bridge siloed health systems.
🔹 Moderna is laying off ~10% of staff, aiming to reduce headcount from 5,800 to under 5,000 as part of a $1.5B cost-cutting plan through 2027, including scaling back late-stage respiratory vaccine programs and manufacturing expenses.
💰 Money Flows
(Funding rounds, IPOs, and M&A for startups and smaller companies)
🔹 Ultromics raises $55M to scale AI heart diagnostics—Oxford-based Ultromics secures $55M in Series C to expand global use of its FDA-cleared AI ultrasound tools for early detection of heart failure and cardiac amyloidosis, including the EchoGo platform trained on over 430,000 scans.
🔹 AI-Native Formation Bio, backed by a $372M Series D, licenses a Phase 1–ready monoclonal antibody for autoimmune diseases from IMIDomics, aiming to enhance its pipeline through AI-integrated development and clinical trial platforms.
🔹 Recursion posts Q2 update with Sanofi milestone, trial progress—techbio company reported a $7M milestone from Sanofi and progress on RBM39 and CDK7 programs, while expanding use of causal AI and generative models; cash runway extends to late 2027 with $533.8M on hand.
🔹 Biogen builds ventures arm, forming a “New Ventures” team to invest in external early-stage programs.
🔹 Frazier closes an oversubscribed $1.3B fund to back early-stage therapeutic companies, bringing total venture capital raised since 2016 to over $3.6B and continuing its hands-on approach to biotech company creation.
🔹 OpenAI-backed Ambience Healthcare raised $243M in Series C funding led by a16z and Oak HC/FT to scale its ambient AI platform that automates clinical documentation and coding across 100+ specialties, now valued at over $1B and adopted by major U.S. health systems.
⚙️ Other Tech
(Innovations across quantum computing, BCIs, gene editing, and more)
🔹 Neuralink begins UK trial of brain implant for paralysis—launching its first UK clinical trial of the fully implantable N1 brain-computer interface, enrolling up to seven participants with paralysis to assess device safety and function at UCLH and Newcastle hospitals.
🔹 Synchron achieves thought control of iPad via brain implant, demonstrating the first native brain-computer interface control of an Apple iPad using its minimally invasive Stentrode implant, enabled by Apple’s new BCI HID protocol and tested in ALS patients as part of the COMMAND trial.
🔹 Neal Amin and Stanford researchers publish results of a 7-year effort in Science, creating the most advanced stem cell-derived model of the human neural midline using floor plate cells and assembled organoids (assembloids) to replicate neural patterning, axon guidance, and circuit formation.
🔹 Nikolai Slavov and colleagues at Parallel Squared Technology Institute are advancing scalable single-cell proteomics using novel barcoding and timePlex methods, with the goal of matching DNA sequencing throughput—efforts reported in Asimov Press and Nature Methods.
🔹 Olympus and Revival Healthcare Capital launch Swan EndoSurgical with $65M to develop a robotic platform for minimally invasive gastrointestinal procedures, with Olympus holding an acquisition option and up to $458M in milestone-based funding.
🏛️ Bioeconomy & Society
(News on centers, regulatory updates, and broader biotech ecosystem developments)
🔹 First EU-approved blood test to rule out Alzheimer’s—Roche secures CE mark under new EU diagnostics rules for a blood test detecting pTau-181, offering a non-invasive tool to exclude Alzheimer’s in primary care with 93.8% negative predictive value.
🔹 ARPA-H launches brain regeneration program—the "FRONT" initiative, led by Jean Hebert—to enable full regeneration of neocortical brain tissue using stem cell scaffolds, targeting conditions like stroke and trauma; initial budget proposed at $110M with proposals due this fall.
🔹 npj Digital Medicine opens call on AI mental health tools—a special collection on AI-enabled therapies for mental health, seeking research on adaptive, autonomous, and human-in-the-loop systems with clinical, ethical, and regulatory evaluation; submissions open through May 2026.
🔹 Gabriele Corso announces a call for proposals to validate Boltz-2’s small-molecule design models through collaborative hit-discovery efforts, offering partners tailored molecule designs in exchange for experimental testing and feedback.
🔹 The White House has abruptly halted all NIH research grants and contracts via an OMB directive, freezing funding to academic and medical institutions nationwide; meanwhile, FDA biologics chief Vinay Prasad suddenly resigns less than three months into his role. As of August 5, the freeze has been lifted. Meanwhile, the Senate Appropriations Committee rejected proposed NIH cuts, advancing a bill to boost the agency’s budget by $400M for FY2026, preserving its 27 institutes and signaling bipartisan support for continued biomedical research funding.
🔹 FDA clears Elevidys for ambulatory DMD patients—the agency recommends lifting the voluntary hold on Sarepta’s gene therapy Elevidys for ambulatory Duchenne muscular dystrophy patients, finding a recent patient death unrelated to the therapy; the hold remains for non-ambulatory patients pending further review.
🚀 A New Kid on the Block
(Emerging startups with a focus on technology)
🔹 YC-backed Fluidize debuts with an AI platform that automates and simplifies R&D workflows, enabling scientists to run simulations and validations via natural language, reducing setup friction and accelerating discovery.
💊 Pharma
🔹 Atai Life Sciences' experimental therapy for cognitive impairment in schizophrenia failed to improve outcomes in a phase 2 trial, leading the company to deprioritize the asset despite its favorable safety profile.
🔹 Meanwhile, MapLight Therapeutics raises $373M in Series D to fund phase 2 trials of its oral M1/M4 muscarinic agonist combo for schizophrenia and Alzheimer’s psychosis, entering the same space as BMS’ approved drug Cobenfy.
🔹 Leads Biolabs goes public in Hong Kong—Chinese antibody developer raises $189M in HKEX IPO to advance its cancer and autoimmune pipeline, with $123M earmarked for clinical trials across its portfolio of monoclonal, bispecific, and ADC therapies.
🔹 Twist Bioscience launches a humanized transgenic mouse model to accelerate in vivo antibody discovery, offering faster, flexible lead generation without licensing hurdles and expanding its existing DiversimAb and B cell screening platforms.
Join us for Under the Hood: The Real Mechanics of AI in Clinical Development, a virtual mini-conference featuring presentations by industry practitioners, building AI-based solutions for clinical research. September 23, 2025, 5:00 - 7:00 PM CET.
Live demos, focused presentations, and clear-eyed discussion — the event will be hosted by Dr. Louise von Stechow and Dr. Andrii Buvailo, and will feature several practically-oriented presentations by invited industry experts and AI tool builders. You will learn what’s working, what still isn’t, and what’s next.
Pathology Foundation Models Are Learning the Wrong Signals
A new preprint out of BIFOLD, LMU Munich, and the Netherlands Cancer Institute makes an observation that most digital pathology foundation models (FMs) are a lot better at recognizing scanner quirks than biological signals.
The team—Hense, Kömen, de Jong, and colleagues—ran a benchmark across 20 popular pathology FMs and found consistent robustness gaps. The models weren’t failing on accuracy per se, but rather on what they were learning: technical artifacts like lab prep style, scanner hardware, or staining variations—none of which should influence diagnosis, but often do in practice.
They introduce PathoROB, a robustness benchmark built from 4 datasets covering 28 biological classes from 34 institutions. It includes new metrics like the robustness index, allowing side-by-side comparison of model performance when irrelevant technical variation is introduced. Spoiler: every FM showed weaknesses.
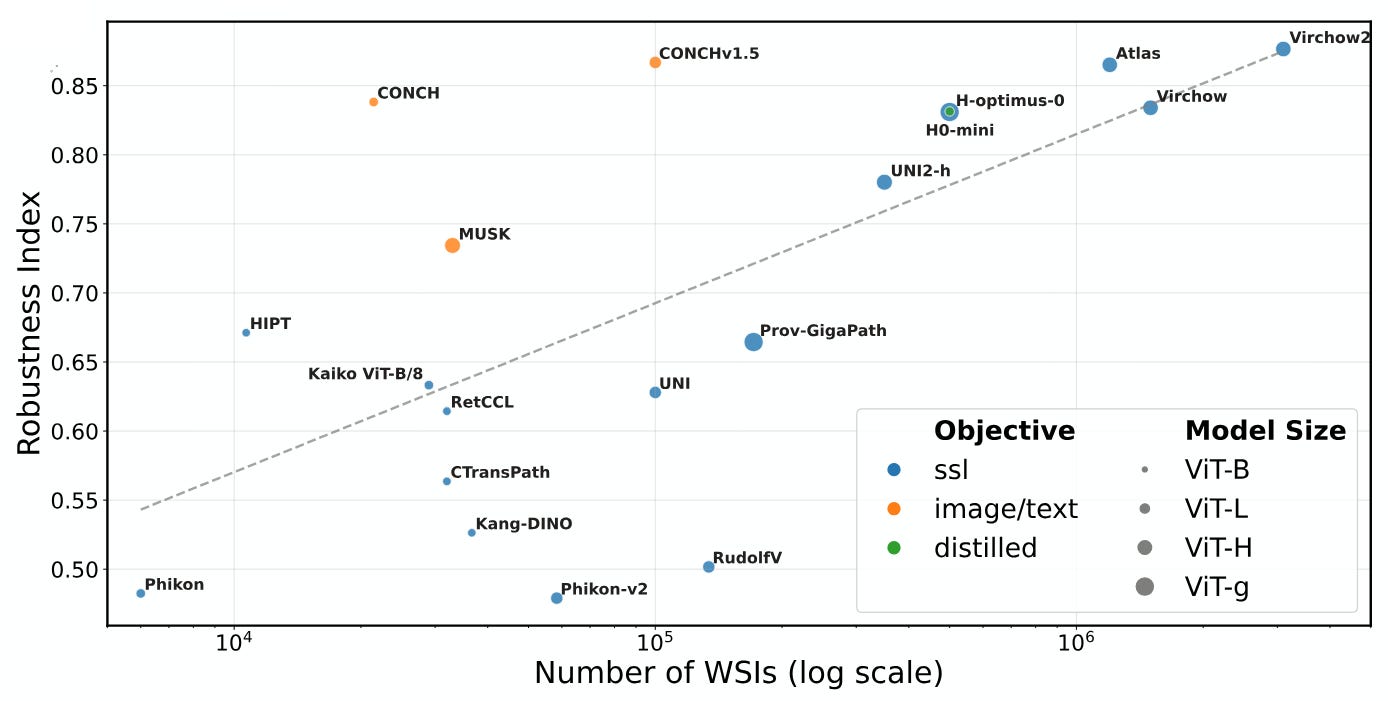
According to authors, downstream consequences aren’t subtle, cluster assignments reflect technical domains instead of biology, and diagnostic pipelines degrade in ways that aren’t immediately obvious. And because many FMs are used as drop-in components downstream, any noise in their representations propagates.
The paper also sketches a fix: post-hoc robustification methods that reduce—but don’t fully eliminate—these issues, even without retraining the FM. Still, the authors argue that robustness needs to be treated as a first-class design constraint going forward.
You can catch more detail in a talk by Edwin de Jong here on YouTube. Public release of the PathoROB benchmark is coming soon.
The Landscape of Gene Delivery: What’s Working, What’s Lagging
We recently came across a comprehensive piece by Eryney Marrogi at Asimov Press that walks through the major gene delivery vectors in use today—viral and non-viral—and how their tradeoffs stack up in practice.
The article covers five main classes: AAV, adenovirus, LNPs, HSV, and lentivirus:
AAV is the current workhorse, with multiple FDA-approved therapies (Zolgensma, Luxturna, Hemgenix). It's small, relatively well tolerated, and can persist in non-dividing cells. But the ~4.7 kb packaging limit forces compromises, especially with tools like Cas9. Engineering efforts—particularly AI-driven capsid design from Dyno and Capsida—are trying to stretch AAV's reach, but immune responses and pre-existing antibodies remain serious bottlenecks.
Adenovirus has a larger cargo capacity (~36 kb), making it attractive for big constructs. However, its history is checkered—Gelsinger’s 1999 death still casts a shadow. Immunogenicity is high, and expression tends to be transient. That said, it’s powering some cancer therapies and has a track record in vaccine platforms (COVID-19, Ebola). Non-human and chimeric capsids may help sidestep immunity issues.
LNPs excel at delivering RNA, are cheap to manufacture, and were central to the success of mRNA COVID-19 vaccines. Intravenously, they accumulate in the liver by default, but newer designs—antibody-conjugated, lipid-tuned, or chemically modified—are broadening their reach. They also support redosing, which viral vectors struggle with.
HSV flies under the radar but brings some unique advantages. Its neurotropism, large packaging capacity (~152 kb), and ability to establish latency in neurons make it a candidate for CNS-targeted therapies. T-VEC (HSV-1–based) is already FDA-approved for melanoma. HSV vectors can travel retrogradely along neurons, opening non-invasive routes into the brain. Still, pre-existing immunity and cytotoxicity are non-trivial concerns.
Lentivirus, derived from HIV, holds the line for ex vivo editing—e.g. CAR-Ts and blood disorders. It integrates into the genome, offering durability, but that also raises concerns about insertional mutagenesis. In vivo applications are still largely theoretical due to safety and manufacturing hurdles.
Marrogi notes that no single vector fits all use cases. Tradeoffs around expression duration, immune profile, packaging capacity, and targeting specificity shape the field. Hybrid systems may help bridge some of these gaps, but for now, each tool remains context-dependent.