Weekly Tech+Bio Highlights #49: Radiology AI Grows, Impact Evidence Lags
~$1.8B in new funds and rounds; Can AI Scientists Help Rare Disease Research? & Key Tension in Applying Virtual Cell Models to Drug Development
Hi! This is BiopharmaTrend’s weekly newsletter, Where Tech Meets Bio, where we explore technologies, breakthroughs, and cutting-edge companies.
If this newsletter is in your inbox, it’s because you subscribed, or someone thought you might enjoy it. In either case, you can subscribe directly by clicking this button:
In this issue: NIH’s GeneAgent tightened genomics interpretation vs. GPT‑4, Stanford–UCSD matched or beat baselines with a generative segmenter trained on as few as 9 examples, and Duke’s foundation model trained on 6.9M MRI slices outperformed SOTA, while a European Radiology audit logged 173 CE‑marked radiology AI products with 66% peer‑reviewed evidence but only ~24% showing clinical/economic impact;
Weizmann reported a 13k‑participant health digital twin across 17 systems; regulators probed a pediatric death after Sarepta’s Elevidys; and capital flowed with ~$1.8B in new funds and rounds (Omega $647M, TCG Labs Soleil $400M, Brandon ~US$290M, Dispatch $216M, Modi $88M, Avalyn $100M), plus Lilly’s deal with Gate Bioscience up to $856M;
Policy momentum grows via the White House “Winning the Race” AI action plan and EU CHMP backing Lilly’s Kisunla and Gilead’s twice‑yearly HIV PrEP injection.
🤖 AI x Bio
(AI applications in drug discovery, biotech, and healthcare)
🔹 Reducing LLM hallucinations in genomics—NIH researchers introduce GeneAgent, an AI tool for gene-set analysis that outperforms GPT-4 by autonomously verifying outputs against biological databases, improving accuracy and reliability in functional genomics interpretation.
🔹 Training clinical AI with just 9 examples—Stanford and UCSD researchers unveil in Nature a generative AI framework that matches or beats baseline segmentation models using 8-20x less labeled data, showing strong generalization and outperforming UNet and DeepLab across 19 datasets and 3D tasks like hippocampus and liver segmentation.
🔹 New foundation model for MRI—Duke researchers (Maciej Mazurowski) release MRI-CORE, a foundation model trained on 6.9 million MRI slices across 107 protocols and 9 sequence types, outperforming state-of-the-art in segmentation and classification tasks.
🔹 Radiology AI market grows, but evidence gaps remain—a European Radiology study led by researchers from Radboud University and partners finds CE-certified radiology AI products rose to 173 by 2023, with peer-reviewed evidence increasing to 66%, yet only ~24% addressed clinical or economic impact—highlighting a persistent need for higher-level validation. Analysis was supported by data from the Health AI Register.
🔹 Weizmann researchers create AI-based digital twin for health prediction—using data from over 13,000 participants in the Human Phenotype Project, the team developed a digital twin model that tracks biological aging and predicts disease risk and treatment effects across 17 body systems.
This newsletter reaches over 9K industry professionals from leading organizations across the globe. Reserve your sponsor slot in one of the upcoming issues.
Contact us at info@biopharmatrend.com
🚜 Market Movers
(News from established pharma and tech giants)
🔹 Sarepta’s Elevidys—The FDA is investigating the death of a Brazilian child following treatment with Sarepta’s gene therapy, prompting a temporary U.S. shipment halt; this follows two other patient deaths, a clinical hold on Sarepta’s LGMD program after a separate fatality, and a formal rejection by Europe’s CHMP, which found no significant clinical benefit in its pivotal trial.
💰 Money Flows
(Funding rounds, IPOs, and M&A for startups and smaller companies)
🔹 Fueling AI–biology convergence, Houston-based Modi Ventures closes $88M second fund to back startups at the intersection of AI, drug discovery, and diagnostics, with early bets in protein engineering, cell therapies, and generative biology; total capital now exceeds $134M.
🔹 UK’s Bitfount raises €6.8M to expand its federated AI platform that lets hospitals and pharma firms analyze patient data—including imaging—without moving it, aiming to cut trial setup delays, reduce screening failures (from 60% to 14% in one study), and meet UK goals to slash trial timelines from 250 to 150 days.
🔹 Fortuna Health raises $18M Series A led by Andreessen Horowitz to scale its AI-powered platform that helps patients and providers navigate Medicaid, as new federal rules put millions at risk of losing coverage.
🔹 Slingshot AI raises $53M for therapy chatbot — backed by a16z, the startup adds $53M to its Series A to launch Ash, an AI therapy app trained on real clinical sessions, bringing total funding to $93M for its psychology-focused foundation model.
🔹 Australian Brandon Capital raises ~$290M for its sixth and largest life sciences fund, backing commercial-stage biotech in Australia and abroad, with support from government entities and investors like Hesta, CSL, and NRFC.
🔹 Lilly eyes new small-molecule class, inks a deal worth up to $856M with Gate Bioscience to develop “molecular gates,” oral drugs that eliminate hard-to-target extracellular proteins.
🔹 San Francisco-based TCG Labs Soleil raises $400M to fund single-asset biotechs through phase 2 proof-of-concept, expanding its R&D footprint to Shanghai and building on a venture-biotech model backed by The Column Group.
🔹 Avalyn targets IPF with inhaled drugs, raises $100M to advance inhaled versions of approved IPF treatments, aiming for better lung delivery and fewer side effects; lead candidate is in a 300-patient phase 2b trial.
🔹 Omega Funds closes $647M Fund VIII—the largest fund to date to invest in U.S. and European life science companies tackling severe diseases, building on a track record of 52 approved products and nearly 100 exits.
🔹 Orthopedic AI medtech heads to IPO—Shoulder Innovations sets terms for $100M+ IPO on the NYSE to expand its shoulder implant and AI-powered surgical planning business, reporting $21M in H1 2025 revenue.
Join us for Under the Hood: The Real Mechanics of AI in Clinical Development, a virtual mini-conference featuring presentations by industry practitioners, building AI-based solutions for clinical research. September 23, 2025, 5:00 - 7:00 PM CET.
Live demos, focused presentations, and clear-eyed discussion — the event will be hosted by Dr. Louise von Stechow and Dr. Andrii Buvailo, and will feature several practically-oriented presentations by invited industry experts and AI tool builders. You will learn what’s working, what still isn’t, and what’s next.
⚙️ Other Tech
(Innovations across quantum computing, BCIs, gene editing, and more)
🔹 Reported first digital twin from organ-on-a-chip—Hesperos creates a validated digital twin of human disease using a multi-organ chip model of malaria, combining human tissues with PK/PD modeling to predict drug responses, advancing non-animal, patient-specific testing.
🔹 Korean CRO enters next-gen preclinical testing—CorestemChemon partners with ATG Lifetech to develop organoid models and transcriptomics-based platforms for liver, heart, and brain drug testing.
🏛️ Bioeconomy & Society
(News on centers, regulatory updates, and broader biotech ecosystem developments)
🔹 Biotech layoffs—Adicet Bio, Rocket Pharmaceuticals, Tessera Therapeutics, Generate:Biomedicines, Genentech, GSK, and Hookipa Pharma all announced layoffs last week.
🔹 White House’s “Winning the Race” plan designates healthcare as a priority sector, launching FDA regulatory sandboxes, AI testbeds, and cloud-enabled biomedical labs to accelerate safe deployment of diagnostics, clinical tools, and AI-driven drug discovery.
🔹 Europe’s CHMP reverses its earlier stance and recommends Eli Lilly’s Kisunla for early Alzheimer’s in patients with low ApoE4 risk, while also endorsing Gilead’s twice-yearly HIV PrEP injection Yeytuo under accelerated review for its global public health potential.
🚀 A New Kid on the Block
🔹 New shot at solid tumors—Dispatch Bio launches with $216M to develop a two-part immunotherapy for solid tumors, combining a viral vector that marks cancer cells with CAR-Ts to clear them, co-founded by CAR-T pioneer Carl June and backed by Arch, PICI, and BMS.
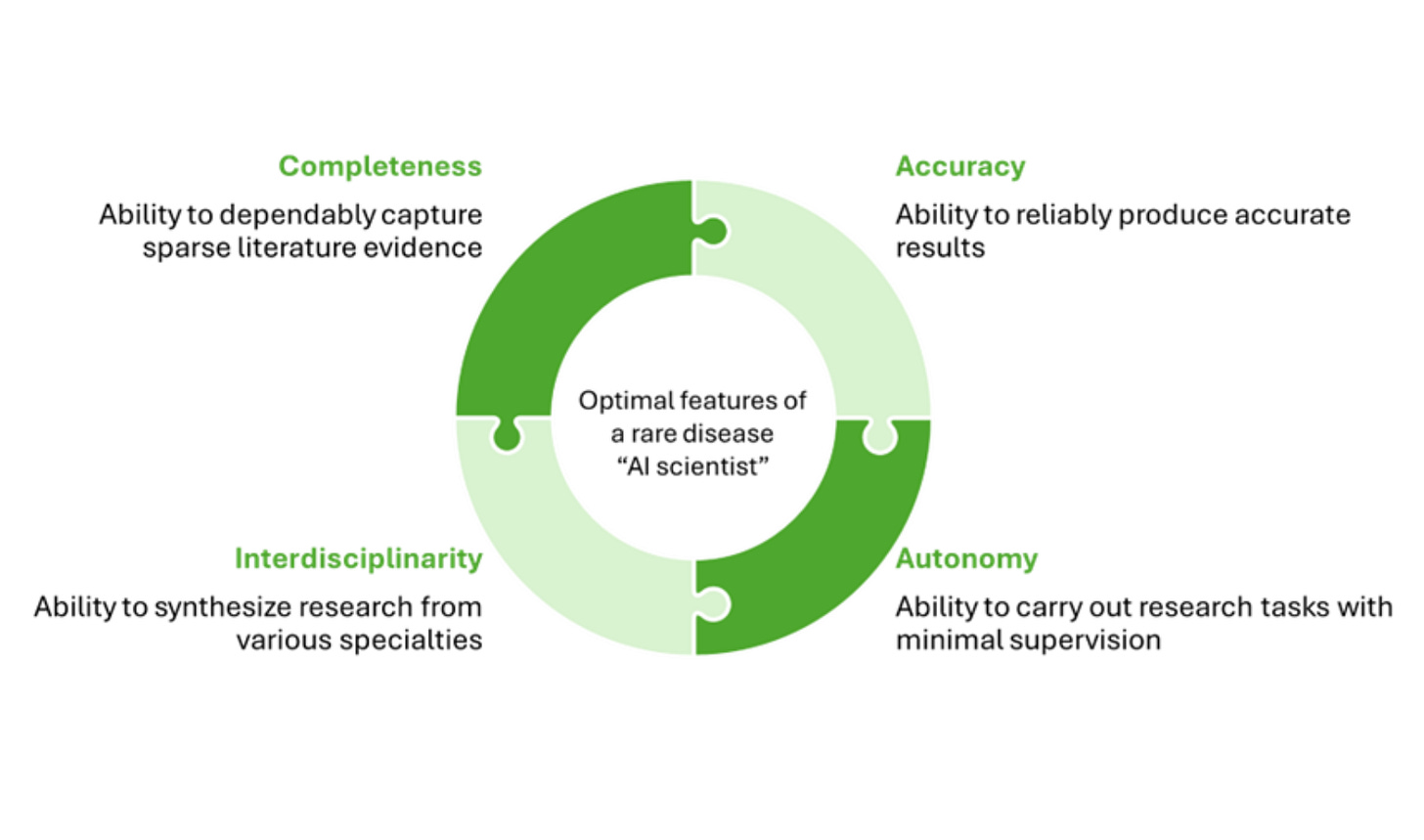
Can "AI Scientists" Help Rare Disease Research?
In her latest Tech for the Rare column, BiopharmaTrend contributor Louise von Stechow explores whether agentic AI systems—often called “AI scientists”—might help rare disease researchers overcome persistent structural barriers: sparse data, limited funding, siloed expertise, and slow discovery timelines.
Unlike conventional AI tools that specialize in narrow tasks, these experimental systems aim to participate in the scientific process itself. Early-stage systems like Google’s Gemini-based co-scientist and Stanford’s GPT-4-based Virtual Lab simulate interdisciplinary collaboration through multi-agent architectures, assigning roles like Principal Investigator, Critic, or domain specialists. The result is a structured, internal debate that outputs hypotheses, experimental plans, or repurposing suggestions.
von Stechow notes that while current deployments remain limited and anecdotal, some early signs are promising—especially in settings where real-world expertise is scarce. In one example, Google's co-scientist proposed a drug repurposing lead for liver fibrosis that had sparse existing literature support. And in a well-known real-world case, researchers affiliated with EveryCure used AI to identify adalimumab as a treatment for a rare subtype of Castleman’s disease—extending the patient’s life by years.
However, technical and conceptual limits remain. These systems still hallucinate, lack temporal and causal understanding, and struggle with prioritizing among competing sources. Their utility in rare disease research may be less about full autonomy and more about acting as amplifiers—surfacing weak signals, generating drafts, or simulating cross-disciplinary input when no such team exists.
von Stechow maps out several rare disease challenges and possible AI functions side by side: knowledge synthesis across modalities, simulating interdisciplinary reasoning, protocol drafting, and low-cost in silico prescreening that might eventually feed into automated lab systems. This modular framing echoes the “lab-in-the-loop” approach described by Genentech’s Aviv Regev, where human researchers supervise iterative AI-experimentation cycles.
It’s unclear whether AI scientists will evolve into trusted research partners or remain productivity tools with strong prompting requirements. But for rare disease—where lack of time, people, and funding are the rule—Louise argues the bar for utility might be lower, and the upside well worth exploring.
Radiology AI Grows, Impact Evidence Lags
Kicky van Leeuwen (AI researcher and managing partner at Romion Health) shared a follow-up to her earlier award-winning study on the first 100 radiology AI tools. The new paper, co-authored with Noa Antonissen, Olga Tryfonos, Ignas Houben, Colin Jacobs, and Maarten de Rooij, surveys how the field has evolved through 2023.
This time, the dataset covers 173 CE-certified AI products from 90 vendors, nearly double the 2020 count. The proportion of products with peer-reviewed evidence rose from 36% to 66%, backed by 639 studies.
However, the type of evidence hasn't shifted much: most publications still focus on diagnostic accuracy (level 2 in the analysis—studies that assess how well the AI performs its technical task), while studies measuring clinical decision-making, patient outcomes, or economic value (levels 3–6—evidence of real-world impact on care decisions, health outcomes, or costs) remain limited—about 24%, unchanged since 2020.
Other trends suggest mixed signals: multicentre studies increased (30% to 41%), but vendor-independent and multinational designs both declined slightly. Prospective studies are still in the minority.
As van Leeuwen notes, this points to a maturing field—but one still anchored in performance metrics. Clinical and cost-effectiveness data, the kind that supports adoption and reimbursement, remain sparse. Without these, many AI tools risk staying in the “nice-to-have” category rather than becoming embedded in clinical decision pathways.
The study, Artificial Intelligence in Radiology: 173 Commercially Available Products and Their Scientific Evidence, is published in European Radiology (July 2025) and draws from data curated in the Health AI Register.
Are Virtual Cells Solving the Wrong Problem?
Patrick Malone, MD PhD—partner at KdT Ventures (portfolio includes PathAI, Dyno Therapeutics, and other biotech startups)—recently pointed out a key tension in applying “virtual cell” models to drug development: the disconnect between the level at which biology is measured and the level at which intervention occurs.
We often model biology at the single-cell level (think scRNA-seq), but real-world interventions play out at the tissue, organ, or patient level. A model might accurately predict that a compound downregulates TGF-β in fibroblasts, but whether that translates into reversing fibrosis in vivo depends on broader systems—drug delivery, extracellular matrix remodeling, immune response, and more.
In short, cell-level predictions can be technically right but clinically irrelevant if they ignore the higher-order context where effects actually manifest.
Some strategies Malone flags as promising to bridge this abstraction gap:
Multiscale modeling: nesting virtual cells inside organ-level simulations
Spatial transcriptomics: keeping the positional data that gives cell behavior meaning [ed: e.g. NOETIK’s “Octopia”]
Surrogate modeling: using outputs from cell models to train predictors for outcomes closer to the clinic (biomarkers, histology, etc.)