Weekly Tech+Bio Highlights #46: New DeepMind AI Predicts How Genes Work
~$725M in Biotech Funding, UK R&D Push, & Do We Still Need Animal Testing?
Hi! This is BiopharmaTrend’s weekly newsletter, Where Tech Meets Bio, where we explore technologies, breakthroughs, and cutting-edge companies.
If this newsletter is in your inbox, it’s because you subscribed, or someone thought you might enjoy it. In either case, you can subscribe directly by clicking this button:
🤖 AI x Bio
(AI applications in drug discovery, biotech, and healthcare)
🔹 DeepMind announces AlphaGenome, an AI tool for decoding genetic variants—the model predicts how DNA mutations affect gene regulation in humans and mice, extending to non-coding regions with million-base input.
🔹 Simulating cells with transformers—Arc Institute CTO Dave Burke announces "State", a virtual cell model using transformer-based AI to predict cellular responses to genetic and drug perturbations. With a $175K prize pool, Arc also launched the Virtual Cell Challenge to benchmark AI models predicting gene expression from CRISPRi perturbations
🔹 Cracking ALS with code—a new £7.5M Longitude Prize on ALS launches to spur AI-driven drug discovery for motor neurone disease, offering staged funding to researchers using machine learning to identify novel treatment targets, backed by MND Association, Nesta, LifeArc, and others.
🔹 Schrödinger’s physics-based computational platform enabled rapid design of MALT1 inhibitor SGR-1505, now granted FDA Fast Track for a rare, slow-growing blood cancer.
🔹 Insilico Medicine doses first patient in Phase 1 trial of its AI-designed MAT2A inhibitor targeting MTAP-deleted solid tumors.
🔹 Ultima Genomics’ ultra-high throughput UG 100 platform will power Regeneron’s 800,000-sample proteomics studies with Geisinger and UK Biobank, showcasing the tech’s efficiency for large-scale biological “counting” applications.
🔹 New Stanford-led benchmark (by Suhana Bedi and Nigam Shah) of 121 real-world clinical tasks shows DeepSeek R1 and o3-mini as top-performing healthcare AI models, with strong results in note-writing and patient messaging, but weaker in admin workflows.
🔹 Sora Neuroscience receives FDA clearance for its AI-powered resting-state fMRI software that maps brain function for surgical planning, enabling plug-and-play integration with hospital imaging systems.
🔹 Chatting with DNA—InstaDeep's (Karim Beguir) ChatNT lands Nature Machine Intelligence cover with a model that lets researchers query DNA, RNA, and proteins using natural language, trained on 600M+ DNA tokens and built to bridge genomics and English through text-to-text translation.
🔹 Elsevier launches Embase AI, a GenAI-powered interface for its biomedical literature database, enabling natural language search and summarization across millions of records
🔹 MAXIOM and DNAthlete partner to fuse genomic testing with adaptive AI for real-time, hyper-personalized health and performance guidance.
🔹 Iambic taps Lambda’s NVIDIA HGX B200 cluster to boost Enchant, its AI model for predicting molecular properties and clinical outcomes; aims to accelerate and derisk early-stage drug discovery.
🔹 Cosmo to pilot Apple Vision Pro integration with Medtronic’s AI-powered GI Genius for VR-assisted colonoscopies, aiming to enhance real-time lesion detection without disrupting physician workflow.
🔹 30 million cells, structured for AI—LatchBio, Miraomics, and Pythia Biosciences launch a 30M-cell atlas across 150 diseases and unveil an agentic AI framework that boosts molecular data curation efficiency 40x, offering a ready-to-use resource for biopharma and AI-driven research.
🚜 Market Movers
(News from established pharma and tech giants)
🔹 Bayer's BlueRock lays off 50 staff and closes Cambridge labs to narrow focus on late-stage cell therapies for Parkinson’s and vision loss, dropping cardiology and immunology efforts amid broader pipeline cuts.
🔹 ProFound Therapeutics, a Flagship Pioneering company, partners with Novartis in a four-year deal to identify novel cardiovascular drug targets using its AI-enabled platform for discovering previously uncharacterized proteins, with $25M upfront and up to $750M in milestones per target.
🔹 J&J launches the Polyphonic AI Fund for Surgery with Nvidia and AWS, offering up to $100K and tech support to startups and researchers building AI tools for surgical care.
🔹 Proteomics heats up—Illumina’s $400M acquisition of Somalogic gives it a late but vertically integrated entry into proteomics, Alex Dickinson notes Olink’s first-mover lead remains strong after its $3B Thermo acquisition, but the strategic race is now wide open.
💰 Money Flows
(Funding rounds, IPOs, and M&A for startups and smaller companies)
🔹 XtalPi signs a letter of intent with Harvard’s Greg Verdine and his biotech DoveTree to co-develop drugs using AI and robotics, with $100M upfront and over $10B in potential milestones.
🔹 AI-native Formation Bio licenses dual JAK/SYK inhibitor to Sanofi via subsidiary Libertas Bio in deal worth up to €545M for a new, undisclosed indication.
🔹 LinkedIn co-founder Reid Hoffman leads a $12M round in Sanmai, a stealth startup building a $500 at-home headset that uses AI-guided ultrasound to non-invasively treat mental health conditions like anxiety, with clinical trials underway.
🔹 Neuron23 raises $96.5M Series D and doses first patient in Phase 2 NEULARK trial testing NEU-411, a brain-penetrant LRRK2 inhibitor for genetically driven Parkinson’s, with topline results expected in 2027.
🔹 Alphabet’s Calico signs $596M licensing deal with China’s Mabwell for a clinical IL-11 mAb targeting idiopathic pulmonary fibrosis (IPF), with $25M upfront and global rights outside greater China.
🔹 Vor Bio pivots from cell therapy to autoimmune drugs, licensing RemeGen’s approved dual-target antibody for $45M upfront and $4B in milestones.
🔹 ForSight Robotics raises $125M in Series B funding to launch human trials of its robotic platform for cataract surgery.
🔹 Revolution Medicines lands up to $2B from Royalty Pharma (including $1.25B in synthetic royalties) to back its RAS(ON) inhibitor for cancer, adding to $2.1B already on hand.
🔹 Certify raises $40M to unify and clean provider data for health plans via AI-powered infrastructure, with funding from Transformation Capital, General Catalyst, Upfront Ventures, and SemperVirens.
🔹 Genome-first AI gets a boost—OutSee raises £1.8M seed round led by Ahren Innovation Capital to scale its predictive genomics platform for uncovering new drug targets in CNS, rare, and metabolic diseases.
🔹 Ontrak Health prices $4M public offering to fund its AI-powered behavioral health platform, which uses predictive analytics and personalized care to reduce costs and improve outcomes for high-need patients.
🔹 Minovia Therapeutics to go public via SPAC merger with Launch One at a $180M valuation, scaling its mitochondrial augmentation therapy; lead program MNV-201 holds FDA Fast Track and Rare Pediatric Disease designations.
⚙️ Other Tech
(Innovations across quantum computing, BCIs, gene editing, and more)
🔹 A glimpse at trisomy reversal in human cells?—in February, Japanese scientists used CRISPR to remove the extra chromosome causing Down syndrome in human lab-grown cells, restoring more typical gene activity and cell function.
🔹 Turning plastic bottles into paracetamol—researchers engineered E. coli to convert PET plastic waste into paracetamol, enabling in vivo drug synthesis from recycled materials.
🔹 Tissium receives FDA de novo clearance for a sutureless, light-activated implant system for peripheral nerve repair—its first U.S. approval, based on a dissolvable biopolymer platform co-invented at MIT.
🏛️ Bioeconomy & Society
(News on centers, regulatory updates, and broader biotech ecosystem developments)
🔹 UK unveils a long-term industrial strategy aiming to become a top 3 global life sciences economy by 2035, committing over £2B ($2.7B) in R&D, health data, genomics, and translational infrastructure, alongside regulatory streamlining and industry partnerships.
🔹 Is Boston losing its biotech crown?—Scott Kirsner reports from the BIO 2025 conference, where industry leaders voice concern over VC pullback, federal cuts, and global competition threatening Boston’s top spot in biotech.
🔹 Global health groups set AI guardrails—six global healthcare bodies add a fifth principle to their joint ethical framework, spotlighting responsible AI and data use to safeguard patient autonomy, accountability, and trust in digital health.
This newsletter reaches over 8.9K industry professionals from leading organizations across the globe. Interested in sponsoring?
Contact us at info@biopharmatrend.com
AlphaGenome Reads 1 Million DNA Letters at Once
DeepMind just introduced AlphaGenome, a new sequence-based model for predicting how mutations in the genome might affect gene regulation. It’s now in research preview (via API), and it builds on earlier efforts like Enformer and AlphaMissense, but tries to unify them—bringing long-range context, base-level resolution, and a broader range of tasks into one model.
The model takes in long DNA sequences (up to a million base pairs) and predicts thousands of molecular readouts—like where transcription starts, how much RNA gets made, which bases are accessible or bound by proteins, or how genes get spliced across different tissues. It can also score the effect of a mutation by comparing predictions on mutated vs. unmutated sequences.
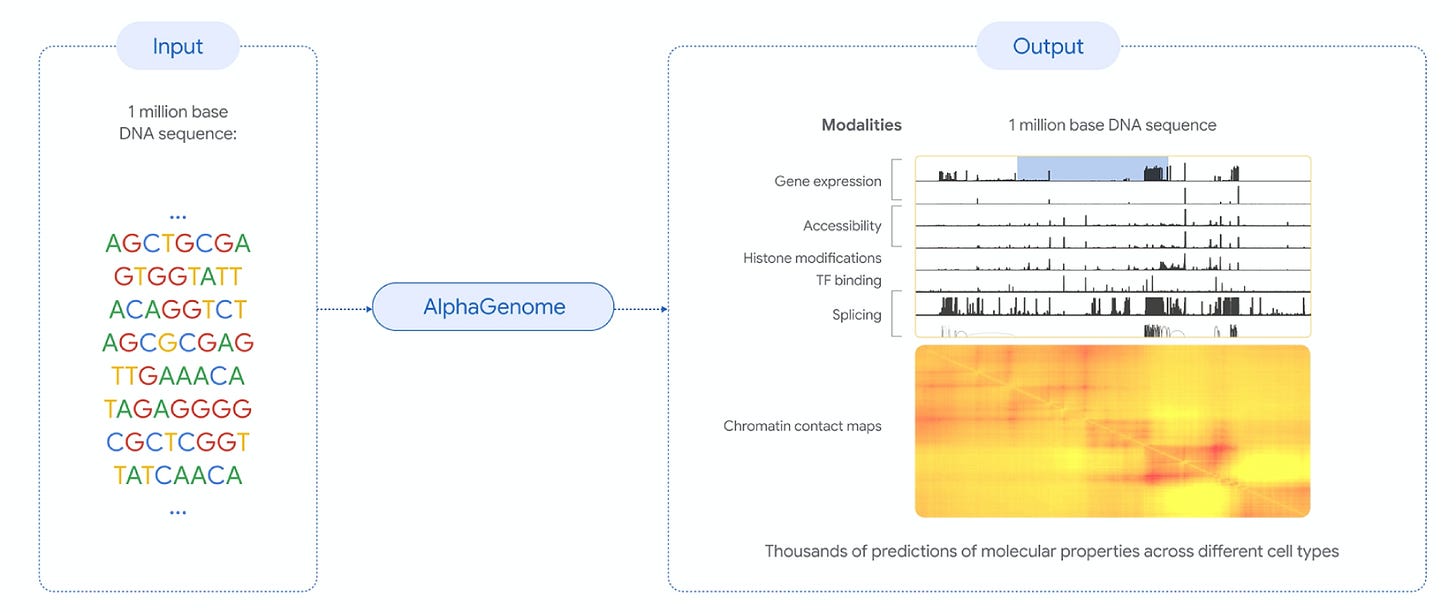
Unlike earlier models that required a tradeoff between long input windows and fine resolution, AlphaGenome handles both simultaneously. It uses a combination of:
convolutional layers (to pick up local motifs),
transformers (to model long-range dependencies),
final prediction heads (to output modality-specific results),
Benchmarks suggest the model performs competitively across multiple prediction tasks—outperforming or matching task-specific models in 24 out of 26 variant effect evaluations, while being the only model to cover all modalities jointly. It also outperformed the best external models on 22 out of 24 single-sequence prediction evaluations.
Notably, it introduces explicit modeling of splice junctions, which may be useful in studying disorders with splicing defects like SMA and cystic fibrosis. Its outputs span a variety of modalities, allowing simultaneous exploration of multiple hypotheses.
Among current limitations is the difficulty in capturing very distant regulatory interactions (>100kb) and the fact that the model is not intended for genome-wide personal prediction or clinical use. The focus remains on characterizing individual variants in research contexts.
The API is available for non-commercial use, and DeepMind is collecting feedback through a community forum.
Do We Still Need Animal Testing?
On a recent episode of WBUR’s On Point, host Meghna Chakrabarti sat down with several key figures in biomedical research to unpack where things stand with alternatives to animal testing. Here goes a brief summary.
The backdrop is the FDA’s recent decision to start phasing out animal testing requirements, beginning with monoclonal antibody therapies, and accelerating review of drug applications that include data from validated non-animal models.
From 1938 to Today
Animal testing has been required by the FDA since a 1938 law passed after the infamous Elixir Sulfanilamide disaster. That law stood for over 80 years, until the 2022 FDA Modernization Act 2.0 opened the door to non-animal alternatives like stem cell models and computer simulations. In early 2025, the FDA formally announced plans to start prioritizing these methods, with monoclonal antibody drugs serving as the first test case.
As Dr. Donald Ingber (Wyss Institute, Harvard) points out, the agency is now offering accelerated review pathways for applications that include data from human-relevant models.
What are the alternatives?
Organs-on-chips and organoids were the two leading technologies discussed in the episode:
Organs-on-chips are thumb-sized microfluidic devices lined with human cells, designed to mimic not just tissue structure but also physiological processes (e.g., breathing motions, blood flow). These systems allow for molecular analysis in real time, including sampling outflows like blood or stool analogs.
See also: Organ-on-a-Chip Companies Usher in a New Era for Drug Trials
“…we get recapitulation of the function or physiology of your organs and disease states at a level really never seen before, and it's all human,” says Ingber.
Organoids are multicellular 3D structures grown from patient-derived stem cells. They often replicate the architecture of specific organs (like brain or intestine), though they typically lack vasculature or immune cell interactions.
“…each one of these has power,” Ingber notes, organoids offer higher throughput and good modeling of cell-level effects; organs-on-chips bring physical realism and dynamic control, including drug dosing that mimics pharmacokinetics.
Is it working?
Some examples: Jenny Tam’s lab (Wyss Institute) uses patient-derived brain organoids to study bipolar disorder, measuring neural activity on microelectrode arrays.
“…we saw that the drug normalized that neural activity back to someone who has not had bipolar,” she said, referring to a candidate identified using her platform.
Meanwhile, Emulate (founded by Ingber) has been validating a liver chip through the FDA’s ISTAND program, testing it on 27 known drugs across chips built from three human donors. Result—the liver chip predicted human toxicity 7-8 times more accurately than standard animal models.
So why not replace animal testing today?
Nicole Kleinstreuer (NIH) says they have “no intention of just phasing out animal studies overnight”. Many models aren’t yet validated for FDA contexts of use. Each organ chip or organoid system needs formal qualification, a slow process, requiring extensive comparative studies.
Birgit Girshick (COO, Charles River) echoed that, calling the current direction a move toward hybrid testing, with organoids and chips running side-by-side with reduced animal use.
“The safety profile of many molecules are still not known. So there's a high risk that once this molecule, once this drug enters a body—and this would be the human body—you don't know what happens. So you don't know if it's accumulating in the thyroid. And if you don't have a platform that can replicate a human body, which would be all 78 organs of a human body, you don't know what other side effects it could have,” Girshick said.
She added that Charles River has already cut its animal usage by 50% in some areas, while building a $200M business line around alternative technologies.
Limits, Economics, and the Political Backdrop
The science is not advancing in isolation, both Ingber and Chakrabarti remarked that federal support is weakening just as alternatives to animal testing are gaining traction.
“We've basically lost a generation ... of scientists in America because it's too much uncertainty,” Ingber said, referring to cuts across NIH, NSF, BARDA, and NASA, some of which halted his lab’s projects on radiation injury and spaceflight adaptation.
According to Ingber, an economic analysis accompanying one liver chip study estimated that its use could save the pharmaceutical industry $2-3 billion annually by preventing late-stage clinical failures. Moderna, for instance, uses human liver chips in place of non-human primates to test mRNA delivery vehicles, reportedly at 1/10th the cost and time.
Closing Thought
Ingber summed up the transitional moment as one of pragmatic acceleration instead of an immediate replacement:
“I think the answer is it’s gonna be these three Rs, like progressively replacing, reducing refining.”
And while the promise of faster, safer, more personalized drug development remains attractive, the future of these technologies also depends on funding, validation pipelines, and regulatory clarity.