Weekly Tech+Bio Highlights #17
ALSO: Flagship Pioneering's New AI Platform; AI-Optimized 3D Printing for Organ Models; How VCs and Pharma Form Novel Business Models; Cell Therapy Developers Should Build Slowly with AI
Hi! I am Andrii Buvailo, and this is my weekly newsletter, ‘Where Tech Meets Bio,’ where I talk about technologies, breakthroughs, and great companies moving the biopharma and medtech industries forward.
If you've received it, then you either subscribed or someone forwarded it to you. If the latter is the case, subscribe by pressing this button:
Now, let’s get to this week’s topics!

In Brief
🔬 A 25-year-old woman with type 1 diabetes became insulin-independent after receiving a stem cell transplant, marking a world-first breakthrough. The reprogrammed cells, derived from her own body, were injected into her abdominal muscles, allowing her to produce insulin naturally within three months. Researchers hope to expand this trial and explore broader applications for stem cell therapies in diabetes treatment.
💰 DCVC Bio III closes at $400M, surpassing its $350M target to fund life sciences companies leveraging AI-driven platforms. The fund will back teams working on breakthroughs in drug discovery, agriculture, and biotechnology. Key portfolio companies include AbCellera, Avicenna, and Umoja Biopharma, advancing treatments for COVID-19, Parkinson’s, and cancer. New investments will target areas like precise radiotherapy and immune disorder management, continuing DCVC Bio's focus on data-driven, computational approaches to innovation in biotech.
🔬 Orion and Aitia have partnered to leverage Aitia's Gemini Digital Twins and Causal AI for discovering novel cancer treatments. This collaboration aims to identify new drug targets and develop candidates for multiple oncology indications. Orion holds exclusive rights to research and commercialize these products, with Aitia eligible for over $10 million per drug target in milestone payments.
🔬 Tempus announced an expanded collaboration with Takeda to enhance oncology R&D using Tempus' multimodal real-world datasets and biological models. The partnership will focus on antibody-drug conjugates, bispecifics, and T-cell therapies. Takeda will use Tempus’ AI platform to analyze de-identified patient data and evaluate drug candidates through organoid models reflecting real-world cancer biology.
🔬 Arctoris partners with Alphabet's Isomorphic Labs to support AI-driven drug discovery using Arctoris' Ulysses® automated platform for generating large-scale protein-ligand interaction datasets, enhancing Isomorphic's drug design models. Miles Congreve, Isomorphic's Chief Scientific Officer, will join Arctoris' Scientific Advisory Board.
💰 UK-based biotech startup 199 Biotechnologies secured $6.5M in seed funding to target age-related diseases using epigenetic reprogramming. The company is leveraging Yamanaka factors as a "biological cheat code" to reverse cellular aging and address cancer, neurodegeneration, and other chronic conditions. With plans to enter human trials within two years, 199 Bio is focusing on speed, aiming for rapid clinical progress in China while advancing partnerships and using both mRNA and viral vectors for therapeutic delivery.
🔬 Scientists at Cold Spring Harbor Laboratory and Weill Cornell Medicine have developed a groundbreaking method using genetic "barcodes" to track the spread of cancer cells, particularly in prostate cancer. This new approach, described in Cancer Discovery, reveals that a small group of aggressive cells are responsible for deadly metastases. The barcoding technology allows for precise mapping of cancer's movement, offering potential for more targeted treatments to prevent cancer from spreading in the future.
💰 Aktis Oncology raised $175 million in an oversubscribed Series B round led by RA Capital Management, with co-leads RTW Investments and Janus Henderson Investors, to advance its radiopharmaceutical pipeline, including its Nectin-4-targeted miniprotein radioconjugate.
🔬 Owkin receives FDA notification for its Phase 1 INVOKE study of OKN4395, a dual EP2 and EP4 prostanoid receptor antagonist, as monotherapy and in combination with pembrolizumab for advanced solid tumors. The study is set to begin enrollment in the US, UK, and Australia in early 2025.
💰 GE HealthCare completed its $51 million acquisition of Intelligent Ultrasound's clinical AI business, which develops AI-driven ultrasound image analysis tools. This acquisition enhances GE's ultrasound portfolio, particularly in improving workflow and ease of use for clinicians and patients.
Spotlight
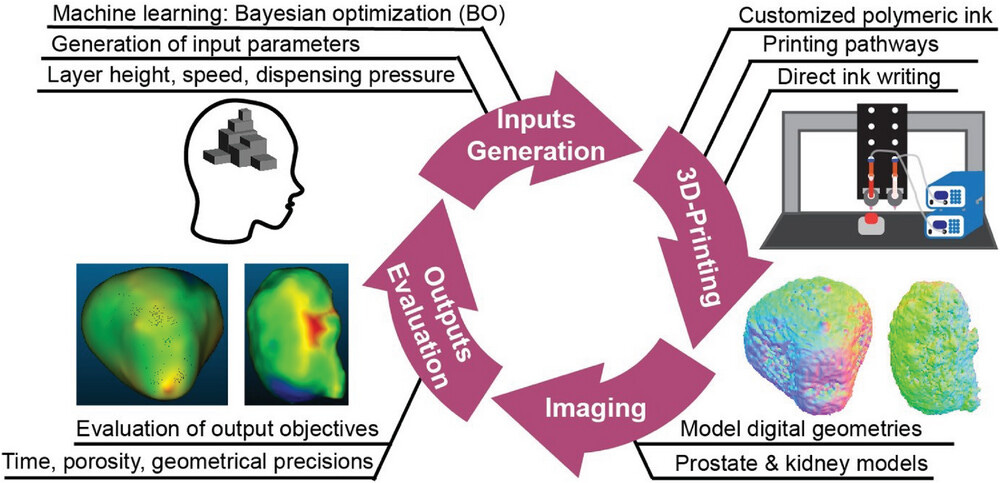
🧬 🖨️ AI-Optimized 3D Printing Transforms Pre-Surgical Practice with Rapid, Precise Organ Models
Researchers at Washington State University (WSU) developed an AI-powered 3D printing system that optimizes organ models for pre-surgical practice. Using a Bayesian Optimization (BO) algorithm, the system fine-tunes printing variables like nozzle size, travel speed, and material pressure to balance speed, accuracy, and material use. The AI improves model fidelity in a recursive process: it prints the model, measures its precision and porosity, and feeds this data back to adjust future prints.
The process leverages Direct-Ink-Writing (DIW) for layer-by-layer printing and NVIDIA GPUs for AI model training. It significantly reduces print time, allowing surgeons to create practice models in just 30 minutes. This technique is also generalizable to other fields like medical devices, aerospace parts, and custom products.
Mirai Bio, launched by Flagship Pioneering, utilizes a machine intelligence-driven platform that integrates in vivo multiplexing and high-throughput automation to generate large datasets for optimizing genetic medicine development. The platform is designed to improve key aspects of genetic therapies, including delivery to specific tissues and cell types, cargo design, and manufacturing processes. It uses proprietary data algorithms to refine and iterate on in vivo tested chemistries, accelerating the development of genetic medicines.
Mirai Bio’s platform focuses on the co-optimization of both cargo design and manufacturing, aiming to improve the success rate and development speed of genetic therapies. The company has received an initial $50 million investment from Flagship Pioneering to advance its platform. Mirai Bio seeks to partner with biotech and pharmaceutical companies, providing an open, end-to-end platform to enhance genetic medicine development across a broad range of therapeutic areas and modalities

R&D Corner
🔬 Even 10 Minutes Matter in Cancer Research
A recent study conducted by Indivumed Therapeutics highlights a crucial, yet often overlooked, factor in cancer research: the cold ischemia time (CIT), or the time it takes to preserve tumor tissues after surgical removal. This seemingly technical detail—whether tissue samples are snap-frozen within 10 minutes or after a longer delay—can profoundly alter the molecular characteristics of the samples, which in turn impacts the discovery of new cancer drug targets.
The findings, published in Cell Death & Disease, reveal that even short delays in preservation can lead to significant shifts in gene expression and protein activity, complicating efforts to accurately identify novel drug targets. This research underscores the importance of rapid tissue handling protocols for ensuring the reliability of molecular data, a critical step in precision oncology.
The research team at Indivumed conducted a multi-omics analysis on more than 1,800 tumor and matched normal tissue samples from patients with colorectal cancer, liver cancer, and two subtypes of lung cancer. They compared the molecular profiles of samples frozen within 10 minutes after removal to those frozen after a delay of 25 minutes or more.
The differences were striking. Samples subjected to longer cold ischemia times exhibited substantial deviations in both gene expression and protein phosphorylation patterns. These changes obscure the true biology of the tumor, leading to potentially misleading conclusions in drug discovery. Gene and protein signatures critical for understanding cancer progression and treatment responses were significantly altered by even small delays, highlighting the fragility of molecular data when tissue is not preserved rapidly enough.
📊 AlphaFold3 Limitations for Antibody Modeling
AlphaFold3 (AF3) has shown progress in antibody and nanobody docking, which is critical for developing antibody therapeutics with high binding affinity and specificity. AF3 achieves 8.9% docking success for antibodies and 13.4% for nanobodies, with a median CDR H3 RMSD accuracy of 2.04 Å for antibodies and 1.14 Å for nanobodies. Importantly, AF3 improves complex prediction accuracy when antigen context is included, particularly for longer CDR H3 loops (over 15 residues). However, AF3 has a 60% failure rate for antibody and nanobody docking when using a single seed, highlighting the need for further improvements to support antibody design and drug development efforts.
The brain microbiome hypothesis suggests that bacteria, fungi, and viruses may exist within the brain, challenging the traditional view of the brain as a sterile organ.
Studies have detected microbial RNA sequences in brain tissues, including in control brains, indicating potential living microorganisms. This concept has implications for neurodegenerative diseases like Alzheimer’s Disease (AD), where specific microbial species have been linked to pathology.
However, the brain microbiome's existence is contested due to concerns about contamination and confounding factors like age-related blood-brain barrier (BBB) deterioration.
The detection methods, which rely on known microbial RNA/DNA sequences, may miss unknown microorganisms, potentially underestimating the brain's microbial diversity. Some theories propose that microbes, particularly from the oral microbiome, may infiltrate the brain via compromised tissues, contributing to amyloid formation in diseases like AD.
Future research must address methodological issues and study diverse age groups to clarify the brain microbiome's presence and significance. Read more here »
Company to Watch
Converge Bio: founded 2024
Antibody humanization refers to the process of modifying an antibody derived from non-human species, typically murine (mouse), to be more structurally and functionally compatible with the human immune system. This is essential for therapeutic applications because non-human antibodies are likely to elicit an immune response when administered to humans, leading to reduced efficacy and potential safety issues. Humanization specifically focuses on altering the framework regions (FRs) of the antibody's variable domains (VH and VL), while maintaining the integrity of the complementarity-determining regions (CDRs), which are responsible for antigen binding.
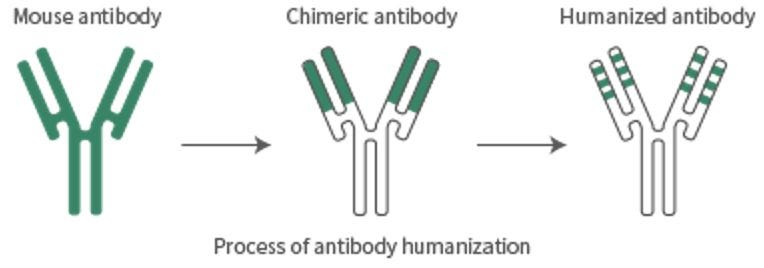
In humanization, computational tools or experimental methods are used to substitute the murine framework residues with human sequences, while ensuring that the CDRs maintain the original antibody's specificity and affinity. This process minimizes immunogenicity by aligning the antibody more closely with the human immunoglobulin repertoire, making it less likely to trigger an immune response.
Large Language Models (LLMs) have emerged as powerful computational tools in this domain. By leveraging vast datasets of antibody sequences and structures, as well as models of human immune responses, LLMs can predict which amino acid substitutions in the antibody framework will successfully humanize the molecule without compromising its antigen-binding function. These models employ advanced machine learning techniques to assess the potential immunogenicity and efficacy of humanized antibodies by evaluating sequence similarities, structural features, and interaction patterns.
The advantage of using LLMs for antibody humanization lies in their ability to process large volumes of data and predict optimal modifications with high accuracy. Traditional methods rely on labor-intensive and time-consuming mutagenesis and screening processes, followed by iterative testing to achieve the desired balance of efficacy and reduced immunogenicity. In contrast, LLMs can perform this analysis in silico, drastically reducing the need for experimental validation cycles, speeding up the preclinical development timeline, and lowering associated costs.
Converge Bio integrates this GenAI-powered LLM technology into its antibody humanization pipeline, offering a more efficient, cost-effective, and precise approach to optimizing therapeutic antibodies for clinical applications.
Takeaways of the Week
💡 New partnership model on the rise: how VCs are helping Pharma hunt for their next blockbuster (Labiotech)
Here’s a concise breakdown of the key ideas:
Pfizer-Flagship Partnership: Pfizer and Flagship Pioneering joined forces to create 10 new drug candidates through a unique “Innovation Supply Chain” model. Pfizer funds programs identified within Flagship’s network of startups, with an option to license or acquire assets.
Strategic R&D Model: Unlike traditional collaborations, this model allows Pfizer to specify desired drugs upfront, and Flagship provides the startups with the technologies to create them. This pre-determined approach accelerates R&D and reduces the time and uncertainty typically involved in such partnerships.
Faster Execution: The established relationship between Pfizer and Flagship allows for rapid startup engagement, speeding up the development process. Two projects—focused on obesity and cardiovascular/renal diseases—have already been announced.
Sector Trend of Outsourcing R&D: This partnership reflects a broader trend where big pharma turns to startups for innovation. Handling R&D internally is costly, and startups often offer more agility and breakthrough potential.
Startups gain early pharma buy-in, reducing risks and securing funding opportunities. They can also quickly assess whether a program aligns with pharma interests, cutting losses on unpromising projects.
The partnership model may inspire similar alliances in the biotech sector, with variations tailored to different companies. Pharma will increasingly rely on external collaborations to access cutting-edge innovation.
💡 Cell Therapy Developers Should Build Slowly with Artificial Intelligence (GEN)
AI in Cell Therapy Manufacturing: Artificial intelligence (AI) can play a vital role in optimizing complex, data-rich processes like cell therapy manufacturing. AI can improve process control, reduce variability, and enhance product consistency, all of which are critical for cell therapy production.
Predictive Maintenance: AI can predict equipment failure, allowing firms to perform maintenance before issues arise, reducing downtime and improving operational efficiency.
Slow Adoption in CGT: Despite its potential, AI adoption in cell and gene therapy (CGT) manufacturing has been slow. While companies see its value, most are still in the early stages of integration due to various challenges.
Infrastructure and Expertise Challenges: Successful AI implementation requires significant investment in infrastructure—real-time data sensors, cloud computing, data management systems, and cybersecurity. Companies must also hire or train AI experts to manage these systems while ensuring compliance with regulatory standards.
Building Slowly: Companies should start small when adopting AI, focusing first on establishing a solid data infrastructure. Pilot projects like predictive maintenance or process optimization offer a lower-risk way to experiment with AI before fully integrating it into manufacturing.
Read also:
14 Foundation Models for Biology Research and Chemistry