Weekly Tech+Bio Highlights #16
Brain Chip Scores FDA’s ‘Breakthrough Device’; Positive Phase IIa Results for AI-Designed Drug; New Letters in Genetic Alphabet; News in Gen AI for Protein Design; The Great Pharma Wasteland
Hi! I am Andrii Buvailo, and this is my weekly newsletter, ‘Where Tech Meets Bio,’ where I talk about technologies, breakthroughs, and great companies moving the biopharma and medtech industries forward.
If you've received it, then you either subscribed or someone forwarded it to you. If the latter is the case, subscribe by pressing this button:
Now, let’s get to this week’s topics!
In Brief
🔬 Insilico Medicine reports positive Phase IIa results for ISM001-055, an AI-designed TNIK inhibitor for idiopathic pulmonary fibrosis (IPF), demonstrating safety and dose-dependent improvements in lung function, marking a significant milestone for AI-driven drug discovery.
🚀 UK-based Constructive Bio has raised $58M to advance its synthetic biology platform, which focuses on rewriting genomes and engineering cell systems for new and existing molecules. Notably, Nobel laureate Venki Ramakrishnan has joined the company's board, bolstering its efforts in developing programmable biomolecules for various industrie
🚀 GC Therapeutics (GCTx), a new cell therapy startup originating from George Church's lab, has raised $75M, including $65M from a recent Series A. The company’s TFome platform aims to revolutionize cell therapy by reducing development time by up to 100 times, leveraging transcription factor biology to streamline the production of induced pluripotent stem cells (iPSCs) for various disease treatments
🔬 Debiopharm partners with WhiteLab Genomics to use AI for enhancing lipid nanoparticle (LNP) targeting in oncology, aiming to improve cancer drug delivery and specificity by identifying cancer-specific receptors and binding agents for LNPs.
🔬 Rakovina Therapeutics partners with Variational AI to develop small-molecule therapies targeting DNA-damage response (DDR) kinases for cancer treatment, leveraging the Enki™ AI platform to identify potential drug candidates.
🔬 DeepCure will present promising preclinical data at the EMBO 2024 Conference on its BRD4 (BD2) inhibitor DC-9476, which showed efficacy in treating macrophage activation syndrome (MAS) by directly inhibiting macrophage activation, outperforming standard glucocorticoid therapy.
🔬 Massive Bio launches "Patient Connect", an AI-powered portal that personalizes cancer clinical trial access by matching patients to trials based on their medical profiles, enhancing accessibility and offering support through concierge services and a clinical network.
💰 Nomic Bio secures $42M in an oversubscribed Series B round to expand its protein profiling platform, nELISA, aiming to accelerate commercial operations and meet growing demand from top pharma and biotech companies for high-throughput proteomics.
🔬 Researchers aboard the ISS are using microgravity to enhance bubble technology for concentrating cancer biomarkers, potentially enabling earlier cancer detection through more sensitive biosensors.
The study led by Professor Tengfei Luo shows that microgravity allows bubbles to grow larger and remain stable longer, improving particle concentration for diagnostic applications.
🚀 Pioneer Group and Novo Nordisk launch the Golden Ticket Programme, offering early-stage biotech companies 12 months of rent-free lab space, expert mentoring from Novo Nordisk, and access to Pioneer’s venture programs. This initiative supports innovations in cardiometabolic health, diabetes, obesity, and rare diseases. Applications close on November 15, 2024.
Spotlight
🧠 Neuralink brain chip for blind people scores FDA’s ‘breakthrough device’ status
Neuralink, Elon Musk’s brain chip company, has received FDA "breakthrough device" status for its experimental brain implant called "Blindsight," aimed at restoring vision for blind patients, including those with damaged optic nerves.
This designation accelerates the regulatory process for innovative devices targeting life-threatening or debilitating conditions, although it doesn’t confirm the device’s safety or effectiveness yet.
The implant, which works by stimulating the visual cortex, is expected to offer initially low-resolution vision, comparable to Atari graphics, but could evolve to surpass natural vision, potentially enabling perception in infrared or ultraviolet light.
Following the August 2024 FDA clearance of its Investigational New Drug (IND) application and Orphan Drug Designation for its TEAD inhibitor ISM6331, Insilico Medicine has now reported promising preliminary results from its Phase IIa clinical trial for ISM001-055, a drug designed to treat idiopathic pulmonary fibrosis (IPF).
ISM001-055, developed using Insilico’s proprietary AI platform, targets TNIK (Traf2- and Nck-interacting kinase), a protein linked to fibrosis, the key pathological process behind IPF. The 12-week Phase IIa trial, which enrolled 71 patients across 21 sites in China, tested the drug’s safety and impact on lung function. The trial met its primary endpoint of safety and tolerability at all dose levels, while also showing a dose-dependent improvement in forced vital capacity (FVC), a critical measure of lung function. Patients receiving the highest dose (60mg once daily) demonstrated the greatest improvement in FVC.
IPF, a rare and progressive lung disease affecting around 5 million people worldwide, has limited treatment options, with current therapies only slowing its progression. ISM001-055, which received FDA Orphan Drug Designation in early 2023, aims to halt or even reverse the fibrosis process in IPF, offering hope for more effective treatment.
This is an important news for AI in drug discovery community, since there are not a lot of AI-designed Phase 2 assets in development out there. So, the results of this program will be a test for the current AI promise, considering that Insilico boasts to have the leading AI platform on the market.
👩🔬 A Milestone for Organ-on-a-Chip Field
Emulate's Liver-Chip has achieved a milestone by being accepted into the Innovative Science and Technology Approaches for New Drugs (ISTAND) Pilot Program.
Notably, it is the first Organ-on-a-Chip technology to receive this recognition. This acceptance signals that the Liver-Chip will now benefit from FDA support as it progresses towards becoming a qualified Drug Development Tool (DDT), aimed at predicting the safety of small-molecule drug candidates during preclinical testing.
As part of the process, Emulate will collaborate with the FDA to develop a qualification plan, advancing to the next stage of the three-phase DDT-qualification process.
R&D Corner
🔬 Scripps Research Scientists Expand the Genetic Alphabet to Create New Proteins
Scripps Research scientists have developed a novel method to incorporate non-canonical amino acids (ncAAs) into proteins using four-nucleotide codons instead of the natural three. This technique enables precise modification of a target gene without altering the entire genome, avoiding genome-wide disruptions.
The method involves utilizing tRNAs assigned to four-nucleotide codons, facilitating ncAA incorporation at specific protein sites, supported by surrounding frequently-used codons.
They successfully engineered more than 100 macrocycles with up to three ncAAs, potentially advancing drug discovery through new-to-nature small molecules. This technique offers an easier and more scalable approach to protein engineering for applications across various sectors.
🔬 Making Skin Transparent Using Common Yellow Food Dye
In a ‘sci-fi’-resembling kind of study, researchers led by Zihao Ou developed a method to achieve optical transparency in live animals by introducing absorbing molecules, such as tartrazine, which enhance transparency in certain wavelengths without increasing light absorption. This approach could significantly improve biological tissue imaging.
🔬 Microsoft’s Foundation Model for Digital Pathology
Somehow I missed this development when it first arrived in May 2024, but it deserves attendion. The article by Microsoft Research describes GigaPath, an advanced vision transformer model developed for digital pathology by researchers from Providence Health System, the University of Washington, and Microsoft Research. This model is specifically designed to handle the large-scale analysis of gigapixel-sized whole-slide pathology images, which are much larger than typical images.
Key features and applications of GigaPath include:
Whole-Slide Modeling: Processes entire gigapixel pathology slides to extract detailed information.
Dilated Self-Attention: Reduces computational complexity, making it possible to analyze such large images efficiently.
Pretrained on Real-World Data: Utilizes a vast dataset of over one billion image tiles from more than 170,000 pathology slides.
It seems GigaPath could be valuable in the field of cancer diagnostics, with applications that include classifying different types of cancer by analyzing whole-slide pathology images and aiding in the determination of cancer stages, which is crucial for treatment planning and outcome prediction.
The model also excels in diagnostic and prognostic prediction, identifying the presence of cancer and other diseases from pathology slides while offering insights into the likely course of the disease.
Additionally, GigaPath supports pathomics by extracting features from pathology images to understand disease mechanisms and identify potential biomarkers. Furthermore, it integrates pathology images with textual data from reports, enhancing the overall diagnostic process through vision-language tasks.
🔬 New AI Model Predicts over 1,000 Diseases Before Diagnosis
I recently read about MILTON, a new machine-learning tool developed by AstraZeneca and detailed in a study published in Nature Genetics.
This tool predicts over 1,000 diseases before diagnosis by analyzing data from the UK Biobank and other biomarkers. Its advanced predictive capabilities are based on routine clinical biomarkers and plasma protein measurements.
MILTON significantly enhances the accuracy of disease prediction using an AUC (area under the curve) metric, with high performance for 1,091 diseases and exceptional performance for 121 diseases. Importantly, it improves the accuracy of genetic discovery by reclassifying potential disease cases that traditional methods might miss.
Company to Watch
Generate:Biomedicines
Massachusetts-based Generate:Biomedicines has announced a multi-target collaboration with Novartis to develop protein therapeutics using its generative AI platform. The deal, valued at over $1 billion with an upfront payment of $65 million, will combine Generate’s AI capabilities with Novartis' expertise in target biology and clinical development. The collaboration aims to develop first- and best-in-class molecules by leveraging AI-based optimization and de novo generation to address multiple disease areas. This is not Generate's first major partnership; its collaboration with Amgen, initiated in 2022, was expanded earlier this year.
At the heart of Generate’s approach is its proprietary Generate Platform, which integrates advanced machine learning models with high-throughput experimental validation to design novel proteins. The platform uses multiparameter co-optimization, allowing the simultaneous optimization of multiple aspects of a therapeutic protein's profile.
For example, the company has successfully developed highly potent monoclonal antibodies, including an anti-IL-13 antibody for treating type-2 inflammation-mediated diseases like atopic dermatitis, as well as an anti-hemagglutinin (HA) antibody targeting influenza.
One of the platform’s major capabilities is its ability to generate de novo binders. These are molecules designed entirely by the computer without any existing templates, enabling Generate to target previously undruggable or hard-to-drug targets with controllable specificity. This technology has already been validated across nine distinct targets, demonstrating superior hit rates compared to traditional methods.
Additionally, Generate has integrated advanced structural biology techniques into its AI learning loop, such as Cryo-Electron Microscopy (CryoEM). This approach generates high-resolution structural data that is used to iteratively train the platform, accelerating the optimization of therapeutic proteins.
Generate is also progressing its in-house clinical pipeline, specifically with the advancement of its Phase 1 study for GB-0669, a monoclonal antibody targeting the S2 stem helix, a highly conserved region of the spike protein in SARS-CoV‑2. The company has demonstrated safety across the first four of five planned cohorts, including the putative recommended dose. This phase 1 trial represents a critical step in evaluating the therapeutic’s efficacy, and analysis of key biomarkers is ongoing. In vitro studies have shown GB-0669 to have potent neutralization effects across all major SARS-CoV-2 variants tested to date, signaling its potential as a broad-spectrum antiviral agent.
Takeaways of the Week
A Downturn of Cell and Gene Therapies
According to a report by BioPharmaDive’s Gwendolyn Wu, cell and gene therapy, once a promising field, is facing a significant downturn in venture capital investment due to manufacturing and drug delivery complexities. Investors are now prioritizing technologies with lower risk and easier market paths, such as small molecules and biologics. This shift reflects challenges in advancing cell and gene therapies despite their potential to offer transformative treatments for conditions like cancer and genetic disorders.
In 2024, cell and gene therapy companies have raised only $500 million across 16 venture rounds, a stark contrast to the $8.2 billion raised across 121 deals in 2021. In 2023, funding had already fallen to $3.5 billion across 65 deals.
Six CAR-T therapies have been approved for cancer treatment, but scaling these treatments, especially allogeneic therapies, has proven challenging due to manufacturing bottlenecks.
Sickle cell gene therapies like Casgevy and Lyfgenia received FDA approvals in 2023, but adoption has been slow, with only a few dozen patients undergoing treatment due to long manufacturing and infusion times.
While some gene therapies like Hemgenix and Roctavian for hemophilia have seen slow uptake, interest remains in autoimmune applications, particularly as treatments that provide durable benefits for patients with no other options.
High costs of manufacturing and complex preconditioning requirements continue to be major barriers for widespread adoption of cell and gene therapies, causing investors to lean towards more predictable biopharmaceutical technologies.
Read the full report for more details »
Current Trends in Brain-Computer Interface (BCI) Research
In his recent LinkedIn post, Prof. Thomas Oxley, CEO of Synchron, commented on a recent FDA-hosted workshop focusing on clinical outcomes in Brain-Computer Interfaces (BCIs).
According to Prof. Oxley, the important takeaways form the workshop are these:
Different BCI approaches target various neurological challenges; no single solution fits all.
Two leading areas of BCI focus are motor and speech restoration.
BCI performance metrics must be directly meaningful for patients' everyday lives.
Motor control is linked to Activities of Daily Living (ADLs), which need validation to show they enhance patient independence.
While speech can be readily measured, outcome measures must connect it to patient autonomy, requiring further validation.
Follow Prof. Thomas Oxley on LinkedIn for regularl insights about BCI progress.
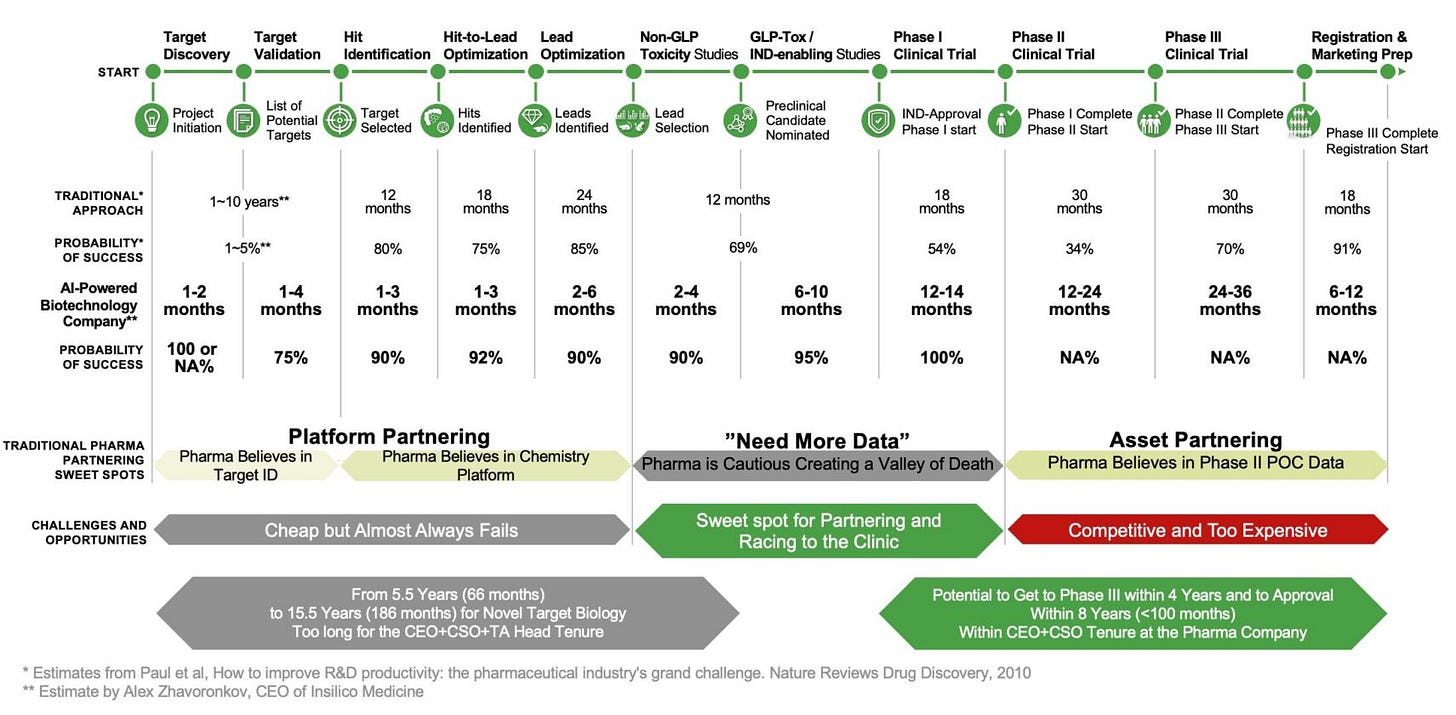
As Dr. Zhavoronkov, co-founder of Insilico Medicine, writes in his GEN article "The Great Pharma Wasteland," the pharmaceutical industry suffers from staggering inefficiency, with drug development costs ranging from $2.8 billion to $6.1 billion per approval and success rates for new drugs remaining low.
He attributes much of this inefficiency to frequent leadership changes, where new CEOs and R&D heads often disrupt ongoing therapeutic programs for strategic reasons unrelated to science or safety. These constant restructurings lead to massive waste, including the abandonment of promising drug candidates.
Dr. Zhavoronkov advocates for long-term commitment from executives and suggests that AI-powered drug discovery companies should aim to advance their programs to at least Phase I before partnering with big pharma to improve chances of success.